Replica Exchange Molecular Dynamics of Diphenylalanine Amyloid Peptides in Electric Fields
- PMID: 33990140
- PMCID: PMC8279545
- DOI: 10.1021/acs.jpcb.1c01939
Replica Exchange Molecular Dynamics of Diphenylalanine Amyloid Peptides in Electric Fields
Abstract
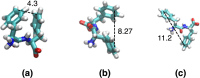
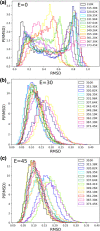
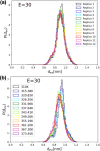
The self-assembling propensity of amyloid peptides such as diphenylalanine (FF) allows them to form ordered, nanoscale structures, with biocompatible properties important for biomedical applications. Moreover, piezoelectric properties allow FF molecules and their aggregates (e.g., FF nanotubes) to be aligned in a controlled way by the application of external electric fields. However, while the behavior of FF nanostructures emerges from the biophysical properties of the monomers, the detailed responses of individual peptides to both temperature and electric fields are not fully understood. Here, we study the temperature-dependent conformational dynamics of FF peptides solvated in explicit water molecules, an environment relevant to biomedical applications, by using an enhanced sampling method, replica exchange molecular dynamics (REMD), in conjunction with applied electric fields. Our simulations highlight and overcome possible artifacts that may occur during the setup of REMD simulations of explicitly solvated peptides in the presence of external electric fields, a problem particularly important in the case of short peptides such as FF. The presence of the external fields could overstabilize certain conformational states in one or more REMD replicas, leading to distortions of the underlying potential energy distributions observed at each temperature. This can be overcome by correcting the REMD initial conditions to include the lower-energy conformations induced by the external field. We show that the converged REMD data can be analyzed using a Markovian description of conformational states and show that a rather complex, 3-state, temperature-dependent conformational dynamics in the absence of electric fields collapses to only one of these states in the presence of the electric fields. These details on the temperature- and electric-field-dependent thermodynamic and kinetic properties of small FF amyloid peptides can be useful in understanding and devising new methods to control their aggregation-prone biophysical properties and, possibly, the structural and biophysical properties of FF molecular nanostructures.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Conformational dynamics and aggregation behavior of piezoelectric diphenylalanine peptides in an external electric field.Biophys Chem. 2015 Jan;196:16-24. doi: 10.1016/j.bpc.2014.08.009. Epub 2014 Sep 7. Biophys Chem. 2015. PMID: 25240398 Free PMC article.
-
Conformational analysis of replica exchange MD: Temperature-dependent Markov networks for FF amyloid peptides.J Chem Phys. 2018 Aug 21;149(7):072323. doi: 10.1063/1.5027580. J Chem Phys. 2018. PMID: 30134732 Free PMC article.
-
Does Replica Exchange with Solute Tempering Efficiently Sample Aβ Peptide Conformational Ensembles?J Chem Theory Comput. 2016 Oct 11;12(10):5201-5214. doi: 10.1021/acs.jctc.6b00660. Epub 2016 Sep 6. J Chem Theory Comput. 2016. PMID: 27560127
-
Influence of various parameters in the replica-exchange molecular dynamics method: Number of replicas, replica-exchange frequency, and thermostat coupling time constant.Biophys Physicobiol. 2018 Aug 8;15:165-172. doi: 10.2142/biophysico.15.0_165. eCollection 2018. Biophys Physicobiol. 2018. PMID: 30250775 Free PMC article. Review.
-
Photoluminescence of Diphenylalanine Peptide Nano/Microstructures: From Mechanisms to Applications.Macromol Rapid Commun. 2017 Nov;38(22). doi: 10.1002/marc.201700370. Epub 2017 Sep 13. Macromol Rapid Commun. 2017. PMID: 28902961 Review.
Cited by
-
Self-assembled short peptides: Recent advances and strategies for potential pharmaceutical applications.Mater Today Bio. 2023 Apr 25;20:100644. doi: 10.1016/j.mtbio.2023.100644. eCollection 2023 Jun. Mater Today Bio. 2023. PMID: 37214549 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

