Fremanezumab for the Preventive Treatment of Migraine: Subgroup Analysis by Number of Prior Preventive Treatments with Inadequate Response
- PMID: 33990144
- PMCID: PMC8411464
- DOI: 10.1177/03331024211008401
Fremanezumab for the Preventive Treatment of Migraine: Subgroup Analysis by Number of Prior Preventive Treatments with Inadequate Response
Abstract
Objective: To evaluate the efficacy of monthly or quarterly fremanezumab in patients with chronic migraine or episodic migraine and documented inadequate response to 2, 3, or 4 classes of prior migraine preventive medications.
Methods: This is an exploratory analysis of a randomized, double-blind, placebo-controlled, phase 3b trial for patients with chronic migraine or episodic migraine and inadequate response to 2 to 4 prior migraine preventive medication classes randomized (1:1:1) to fremanezumab (quarterly or monthly) or placebo. In this exploratory analysis, changes from baseline in the monthly average number of migraine days during 12 weeks of double-blind treatment and adverse events were evaluated for predefined subgroups of patients by number of prior preventive medication classes with inadequate response.
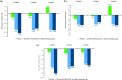
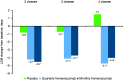
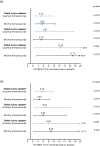
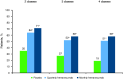
Results: Overall, 414, 265, and 153 patients had inadequate response to 2, 3, and 4 preventive medication classes, respectively. Changes from baseline in monthly average migraine days during 12 weeks were significantly greater with fremanezumab compared with placebo for patients with documented inadequate response to 2 classes (least-squares mean difference vs placebo [95% confidence interval]: quarterly, -2.9 [-3.83, -1.98]; monthly, -3.7 [-4.63, -2.75]), 3 classes (quarterly, -3.3 [-4.65, -1.95]; monthly, -3.0 [-4.25, -1.66]), and 4 classes (quarterly, -5.3 [-7.38, -3.22]; monthly, -5.4 [-7.35, -3.48]) of migraine preventive medications (all p < 0.001). No significant treatment-by-subgroup interactions were observed for any outcome (p interaction > 0.20 for all). Adverse events were comparable for placebo and fremanezumab.
Conclusion: Significant improvements in efficacy were observed with fremanezumab compared with placebo, even in patients who had previously experienced inadequate response to 4 different classes of migraine preventive medications.ClinicalTrials.gov identifier: NCT03308968.
Keywords: CGRP; Chronic migraine; episodic migraine; treatment failure.
Conflict of interest statement
Figures
References
-
- American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache 2019; 59: 1–18. - PubMed
-
- Reuter U.A review of monoclonal antibody therapies and other preventative treatments in migraine. Headache 2018; 58: 48–59. - PubMed
-
- Bigal ME, Lipton RB.Overuse of acute migraine medications and migraine chronification. Curr Pain Headache Rep 2009; 13: 301–307. - PubMed
-
- Messali A, Sanderson JC, Blumenfeld AM, et al. Direct and indirect costs of chronic and episodic migraine in the United States: a web-based survey. Headache 2016; 56: 306–322. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

