Bendamustine-induced nephrogenic diabetes insipidus - A case report
- PMID: 33990157
- PMCID: PMC8685724
- DOI: 10.1177/10781552211013878
Bendamustine-induced nephrogenic diabetes insipidus - A case report
Abstract
Introduction: In patients with relapsed or refractory lymphoma, high-dose chemoimmunotherapy with subsequent autologous hematopoietic cell transplantation (HCT) is a standard of care. Bendamustine, an alkylating agent, is used in the BeEAM (bendamustine, etoposide, cytarabine, melphalan) protocol for conditioning therapy before autologous HCT in patients with relapsed or refractory lymphoma who are eligible for transplant. There is no consensus regarding an optimal salvage regimen and the approach varies according to toxicity.
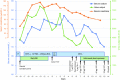
Case report: We present a case of partial nephrogenic diabetes insipidus after receiving bendamustine, as part of the BeEAM protocol.Management and outcome: The patient was managed with parenteral fluid administration and intranasal desmopressin before the condition resolved on its own.
Discussion: We summarize published reports of bendamustine-induced diabetes insipidus.
Keywords: Bendamustine; adverse effect; diabetes insipidus; nephrogenic diabetes insipidus.
Conflict of interest statement
Figures
References
-
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013; 381: 1203–1210. - PubMed
-
- Garnock-Jones KP. Bendamustine: a review of its use in the management of indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. Drugs 2010; 70: 1703–1718. - PubMed
-
- Visani G, Malerba L, Stefani PM, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood 2011; 118: 3419–3425. - PubMed
-
- Kumar L, Ganessan P, Ghosh I, et al. Autologous blood stem cell transplantation for hodgkin and non-Hodgkin lymphoma: complications and outcome. Natl Med J India 2010; 23: 330–335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical