Presymptomatic treatment of classic late-infantile neuronal ceroid lipofuscinosis with cerliponase alfa
- PMID: 33990214
- PMCID: PMC8120778
- DOI: 10.1186/s13023-021-01858-6
Presymptomatic treatment of classic late-infantile neuronal ceroid lipofuscinosis with cerliponase alfa
Abstract
Background: Neuronal ceroid lipofuscinosis type 2 (CLN2 disease) is a rare rapidly progressive neurodegenerative disorder, resulting in early death. Intracerebroventricular enzyme replacement therapy (ERT) with cerliponase alfa is now available and has shown to delay disease progression in symptomatic patients. It is yet unknown if cerliponase alfa can prevent disease onset in presymptomatic patients.
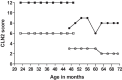
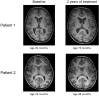
Results: We evaluated the effect of 2 years of intracerebroventricular ERT in two siblings with CLN2 disease, one symptomatic (age 47 months) and one presymptomatic (age 23 months) at treatment start, using the CLN2 Clinical Rating Scale (CLN2 CRS), Gross Motor Function Measure-66 (GMFM-66) for motor function, Bayley Scales of Infant and Toddler Development, 3rd Edition, Dutch (BSID-III-NL) for neurocognitive development, brain MRI, and visual evoked potentials (VEP), electroretinogram (ERG) and retinoscopy for visual function. On the CLN2 CRS patient 1 showed a decline from 3 to 2 in the combined motor and language score due to regression in language use (CLN2 CRS total score after 2 years of treatment: 8), whereas a decline of 2 or more points in the combined motor and language score would be expected without treatment. Patient 2 retained the maximum score of 3 in all 4 subdomains (CLN2 CRS total score after 2 years of treatment: 12). The GMFM-66 total score declined from 46 to 39 in patient 1 and showed an age-appropriate increase from 66 to 84 in patient 2. Cognitive-developmental age decreased from 24 to 11 months in patient 1, whereas an increase in cognitive-developmental age from 21 to 39 months was seen in patient 2. Cerebral and cerebellar atrophy observed on MRI in patient 1 at age 42 months (before treatment) was not observed in patient 2 at age 48 months (after 2 years of treatment).
Conclusion: We show that cerliponase alfa is able to delay the onset of symptoms when treatment is started in a presymptomatic stage of CLN2 disease. Our results advocate the start of treatment at an early age before symptom onset, but should be confirmed in a larger cohort study.
Keywords: CLN2 disease; Cerliponase alfa; Enzyme replacement therapy; Intracerebroventricular; Late-infantile neuronal ceroid lipofuscinosis; Lysosomal storage disorder; Presymptomatic; Tripeptidyl peptidase.
Conflict of interest statement
HHH has received a speaker fee, a participation fee for an advisory board and reimbursement for the ongoing participation in the 190–504 registry study from BioMarin International Limited under an agreement between BioMarin International Limited and Erasmus MC University Medical Center. BioMarin International Limited was not involved at any stage of the study or during the preparation of the manuscript. All other authors declare no competing interests.
Figures

Similar articles
-
Cerliponase alfa changes the natural history of children with neuronal ceroid lipofuscinosis type 2: The first French cohort.Eur J Paediatr Neurol. 2021 Jan;30:17-21. doi: 10.1016/j.ejpn.2020.12.002. Epub 2020 Dec 8. Eur J Paediatr Neurol. 2021. PMID: 33348105
-
Cerliponase Alfa for the Treatment of Atypical Phenotypes of CLN2 Disease: A Retrospective Case Series.J Child Neurol. 2021 May;36(6):468-474. doi: 10.1177/0883073820977997. Epub 2020 Dec 23. J Child Neurol. 2021. PMID: 33356800 Free PMC article.
-
Review of Cerliponase Alfa: Recombinant Human Enzyme Replacement Therapy for Late-Infantile Neuronal Ceroid Lipofuscinosis Type 2.J Child Neurol. 2020 Apr;35(5):348-353. doi: 10.1177/0883073819895694. Epub 2019 Dec 29. J Child Neurol. 2020. PMID: 31884868 Review.
-
Safety and efficacy of cerliponase alfa in children with neuronal ceroid lipofuscinosis type 2 (CLN2 disease): an open-label extension study.Lancet Neurol. 2024 Jan;23(1):60-70. doi: 10.1016/S1474-4422(23)00384-8. Lancet Neurol. 2024. PMID: 38101904
-
Enzyme Replacement Therapy in CLN2-Associated Retinopathy.Klin Monbl Augenheilkd. 2025 Mar;242(3):213-218. doi: 10.1055/a-2528-7886. Epub 2025 Mar 24. Klin Monbl Augenheilkd. 2025. PMID: 40127655 Review. English.
Cited by
-
Embracing the future: Neonatal screening for epileptic syndromes.Epilepsia. 2025 Jun;66(6):1843-1853. doi: 10.1111/epi.18285. Epub 2025 Apr 7. Epilepsia. 2025. PMID: 40192341 Free PMC article. No abstract available.
-
Speech, Language and Non-verbal Communication in CLN2 and CLN3 Batten Disease.J Inherit Metab Dis. 2025 Jan;48(1):e12838. doi: 10.1002/jimd.12838. J Inherit Metab Dis. 2025. PMID: 39821609 Free PMC article.
-
Repositioning Drugs for Rare Diseases Based on Biological Features and Computational Approaches.Healthcare (Basel). 2022 Sep 16;10(9):1784. doi: 10.3390/healthcare10091784. Healthcare (Basel). 2022. PMID: 36141396 Free PMC article.
-
Characterization of neurological disease progression in a canine model of CLN5 neuronal ceroid lipofuscinosis.Dev Neurobiol. 2022 May;82(4):326-344. doi: 10.1002/dneu.22878. Epub 2022 Apr 28. Dev Neurobiol. 2022. PMID: 35427439 Free PMC article.
-
Case Report: The window that closed too soon: lessons from a late CLN2 diagnosis and death of a 9-year-old boy.Front Genet. 2025 Jul 4;16:1622185. doi: 10.3389/fgene.2025.1622185. eCollection 2025. Front Genet. 2025. PMID: 40686560 Free PMC article.
References
-
- Sleat DE, Gin RM, Sohar I, Wisniewski K, Sklower-Brooks S, Pullarkat RK, et al. Mutational analysis of the defective protease in classic late-infantile neuronal ceroid lipofuscinosis, a neurodegenerative lysosomal storage disorder. Am J Hum Genet. 1999;64(6):1511–1523. doi: 10.1086/302427. - DOI - PMC - PubMed
-
- Nickel M, Simonati A, Jacoby D, Lezius S, Kilian D, Van de Graaf B, et al. Disease characteristics and progression in patients with late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease: an observational cohort study. Lancet Child Adolesc Health. 2018;2(8):582–590. doi: 10.1016/S2352-4642(18)30179-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

