An Exposed Outer Membrane Hemin-Binding Protein Facilitates Hemin Transport by a TonB-Dependent Receptor in Riemerella anatipestifer
- PMID: 33990314
- PMCID: PMC8276807
- DOI: 10.1128/AEM.00367-21
An Exposed Outer Membrane Hemin-Binding Protein Facilitates Hemin Transport by a TonB-Dependent Receptor in Riemerella anatipestifer
Abstract
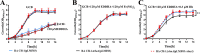
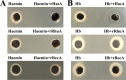
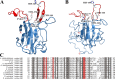
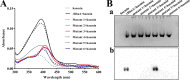
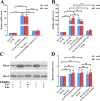
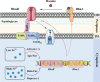
Iron is an essential element for the replication of most bacteria, including Riemerella anatipestifer, a Gram-negative bacterial pathogen of ducks and other birds. R. anatipestifer utilizes hemoglobin-derived hemin as an iron source; however, the mechanism by which this bacterium acquires hemin from hemoglobin is largely unknown. Here, rhuA disruption was shown to impair iron utilization from duck hemoglobin in R. anatipestifer CH-1. Moreover, the putative lipoprotein RhuA was identified as a surface-exposed, outer membrane hemin-binding protein, but it could not extract hemin from duck hemoglobin. Mutagenesis studies showed that recombinant RhuAY144A, RhuAY177A, and RhuAH149A lost hemin-binding ability, suggesting that amino acid sites at tyrosine 144 (Y144), Y177, and histidine 149 (H149) are crucial for hemin binding. Furthermore, rhuR, the gene adjacent to rhuA, encodes a TonB2-dependent hemin transporter. The function of rhuA in duck hemoglobin utilization was abolished in the rhuR mutant strain, and recombinant RhuA was able to bind the cell surface of R. anatipestifer CH-1 ΔrhuA rather than R. anatipestifer CH-1 ΔrhuR ΔrhuA, indicating that RhuA associates with RhuR to function. The sequence of the RhuR-RhuA hemin utilization locus exhibits no similarity to those of characterized hemin transport systems. Thus, this locus is a novel hemin uptake locus with homologues distributed mainly in the Bacteroidetes phylum. IMPORTANCE In vertebrates, hemin from hemoglobin is an important iron source for infectious bacteria. Many bacteria can obtain hemin from hemoglobin, but the mechanisms of hemin acquisition from hemoglobin differ among bacteria. Moreover, most studies have focused on the mechanism of hemin acquisition from mammalian hemoglobin. In this study, we found that the RhuR-RhuA locus of R. anatipestifer CH-1, a duck pathogen, is involved in hemin acquisition from duck hemoglobin via a unique pathway. RhuA was identified as an exposed outer membrane hemin-binding protein, and RhuR was identified as a TonB2-dependent hemin transporter. Moreover, the function of RhuA in hemoglobin utilization is RhuR dependent and not vice versa. The homologues of RhuR and RhuA are widely distributed in bacteria in marine environments, animals, and plants, representing a novel hemin transportation system of Gram-negative bacteria. This study not only was important for understanding hemin uptake in R. anatipestifer but also enriched the knowledge about the hemin transportation pathway in Gram-negative bacteria.
Keywords: Riemerella anatipestifer; hemin-binding protein.
Figures






Similar articles
-
Functional characterization of RhuB as a second TonB2-dependent hemin receptor in Riemerella anatipestifer CH-1.Microbiol Spectr. 2024 Apr 2;12(4):e0313323. doi: 10.1128/spectrum.03133-23. Epub 2024 Feb 20. Microbiol Spectr. 2024. PMID: 38376226 Free PMC article.
-
TonB Energy Transduction Systems of Riemerella anatipestifer Are Required for Iron and Hemin Utilization.PLoS One. 2015 May 27;10(5):e0127506. doi: 10.1371/journal.pone.0127506. eCollection 2015. PLoS One. 2015. PMID: 26017672 Free PMC article.
-
The role of TonB-dependent receptor TbdR1 in Riemerella anatipestifer in iron acquisition and virulence.Vet Microbiol. 2013 Dec 27;167(3-4):713-8. doi: 10.1016/j.vetmic.2013.08.020. Epub 2013 Sep 2. Vet Microbiol. 2013. PMID: 24075356
-
Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii.Biometals. 2009 Feb;22(1):23-32. doi: 10.1007/s10534-008-9202-3. Epub 2009 Jan 7. Biometals. 2009. PMID: 19130255 Review.
-
Iron acquisition in the dental pathogen Actinobacillus actinomycetemcomitans: what does it use as a source and how does it get this essential metal?Biometals. 2007 Jun;20(3-4):365-77. doi: 10.1007/s10534-006-9058-3. Epub 2007 Jan 6. Biometals. 2007. PMID: 17206384 Review.
Cited by
-
The chaperone DnaK promotes the emergence and accumulation of antibiotic-resistant clones through its ATPase activity in Riemerella anatipestifer.Poult Sci. 2025 Jul 3;104(10):105513. doi: 10.1016/j.psj.2025.105513. Online ahead of print. Poult Sci. 2025. PMID: 40675003 Free PMC article.
-
Functional characterization of RhuB as a second TonB2-dependent hemin receptor in Riemerella anatipestifer CH-1.Microbiol Spectr. 2024 Apr 2;12(4):e0313323. doi: 10.1128/spectrum.03133-23. Epub 2024 Feb 20. Microbiol Spectr. 2024. PMID: 38376226 Free PMC article.
-
Functional characterization of two TolC in the resistance to drugs and metals and in the virulence of Riemerella anatipestifer.Appl Environ Microbiol. 2023 Dec 21;89(12):e0130823. doi: 10.1128/aem.01308-23. Epub 2023 Dec 1. Appl Environ Microbiol. 2023. PMID: 38038982 Free PMC article.
-
Manganese Efflux Achieved by MetA and MetB Affects Oxidative Stress Resistance and Iron Homeostasis in Riemerella anatipestifer.Appl Environ Microbiol. 2023 Mar 29;89(3):e0183522. doi: 10.1128/aem.01835-22. Epub 2023 Feb 23. Appl Environ Microbiol. 2023. PMID: 36815770 Free PMC article.
-
Unveiling the silent threat: A comprehensive review of Riemerella anatipestifer - From pathogenesis to drug resistance.Poult Sci. 2025 Apr;104(4):104915. doi: 10.1016/j.psj.2025.104915. Epub 2025 Feb 22. Poult Sci. 2025. PMID: 40020410 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

