The structure of an infectious immature flavivirus redefines viral architecture and maturation
- PMID: 33990320
- PMCID: PMC8121421
- DOI: 10.1126/sciadv.abe4507
The structure of an infectious immature flavivirus redefines viral architecture and maturation
Abstract
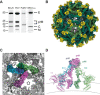
Flaviviruses are the cause of severe human diseases transmitted by mosquitoes and ticks. These viruses use a potent fusion machinery to enter target cells that needs to be restrained during viral assembly and egress. A molecular chaperone, premembrane (prM) maintains the virus particles in an immature, fusion-incompetent state until they exit the cell. Taking advantage of an insect virus that produces particles that are both immature and infectious, we determined the structure of the first immature flavivirus with a complete spike by cryo-electron microscopy. Unexpectedly, the prM chaperone forms a supporting pillar that maintains the immature spike in an asymmetric and upright state, primed for large rearrangements upon acidification. The collapse of the spike along a path defined by the prM chaperone is required, and its inhibition by a multivalent immunoglobulin M blocks infection. The revised architecture and collapse model are likely to be conserved across flaviviruses.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Cammisa-Parks H., Cisar L. A., Kane A., Stollar V., The complete nucleotide sequence of cell fusing agent (CFA): Homology between the nonstructural proteins encoded by CFA and the nonstructural proteins encoded by arthropod-borne flaviviruses. Virology 189, 511–524 (1992). - PubMed
-
- Hobson-Peters J., Harrison J. J., Watterson D., Hazlewood J. E., Vet L. J., Newton N. D., Warrilow D., Colmant A. M. G., Taylor C., Huang B., Piyasena T. B. H., Chow W. K., Setoh Y. X., Tang B., Nakayama E., Yan K., Amarilla A. A., Wheatley S., Moore P. R., Finger M., Kurucz N., Modhiran N., Young P. R., Khromykh A. A., Bielefeldt-Ohmann H., Suhrbier A., Hall R. A., A recombinant platform for flavivirus vaccines and diagnostics using chimeras of a new insect-specific virus. Sci. Transl. Med. 11, eaax7888 (2019). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

