Tumor Immunity and Immunotherapy for HPV-Related Cancers
- PMID: 33990345
- PMCID: PMC8338882
- DOI: 10.1158/2159-8290.CD-20-1760
Tumor Immunity and Immunotherapy for HPV-Related Cancers
Abstract
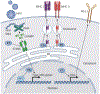
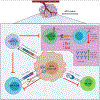
Human papillomavirus (HPV) infection drives tumorigenesis in the majority of cervical, oropharyngeal, anal, and vulvar cancers. Genetic and epidemiologic evidence has highlighted the role of immunosuppression in the oncogenesis of HPV-related malignancies. Here we review how HPV modulates the immune microenvironment and subsequent therapeutic implications. We describe the landscape of immunotherapies for these cancers with a focus on findings from early-phase studies exploring antigen-specific treatments, and discuss future directions. Although responses across these studies have been modest to date, a deeper understanding of HPV-related tumor biology and immunology may prove instrumental for the development of more efficacious immunotherapeutic approaches. SIGNIFICANCE: HPV modulates the microenvironment to create a protumorigenic state of immune suppression and evasion. Our understanding of these mechanisms has led to the development of immunomodulatory treatments that have shown early clinical promise in patients with HPV-related malignancies. This review summarizes our current understanding of the interactions of HPV and its microenvironment and provides insight into the progress and challenges of developing immunotherapies for HPV-related malignancies.
©2021 American Association for Cancer Research.
Conflict of interest statement
Conflicts of interests:
No relevant conflicts of interest to the present publication. Authors receive research funding from Bristol Myers Squibb, Pfizer, Repare therapeutics as well as honoraria from Illumina and repare therapeutics
Figures

Similar articles
-
The Display between HPV Infection and Host Immunity in Cervical Cancer.Front Biosci (Landmark Ed). 2024 Dec 24;29(12):426. doi: 10.31083/j.fbl2912426. Front Biosci (Landmark Ed). 2024. PMID: 39735976 Review.
-
Myeloid Cells Orchestrate Systemic Immunosuppression, Impairing the Efficacy of Immunotherapy against HPV+ Cancers.Cancer Immunol Res. 2020 Jan;8(1):131-145. doi: 10.1158/2326-6066.CIR-19-0315. Epub 2019 Nov 26. Cancer Immunol Res. 2020. PMID: 31771984 Free PMC article.
-
Advances in Designing and Developing Vaccines, Drugs and Therapeutic Approaches to Counter Human Papilloma Virus.Front Immunol. 2018 Nov 12;9:2478. doi: 10.3389/fimmu.2018.02478. eCollection 2018. Front Immunol. 2018. PMID: 30483247 Free PMC article. Review.
-
Regulation of immune responses to HPV infection and during HPV-directed immunotherapy.Immunol Rev. 2011 Jan;239(1):85-98. doi: 10.1111/j.1600-065X.2010.00966.x. Immunol Rev. 2011. PMID: 21198666 Review.
-
ADXS-HPV: a therapeutic Listeria vaccination targeting cervical cancers expressing the HPV E7 antigen.Hum Vaccin Immunother. 2014;10(11):3190-5. doi: 10.4161/hv.34378. Hum Vaccin Immunother. 2014. PMID: 25483687 Free PMC article. Review.
Cited by
-
Review of biomarkers for response to immunotherapy in HNSCC microenvironment.Front Oncol. 2023 Feb 13;13:1037884. doi: 10.3389/fonc.2023.1037884. eCollection 2023. Front Oncol. 2023. PMID: 36860322 Free PMC article. Review.
-
Immune checkpoint blockade for locally advanced or recurrent/metastatic cervical cancer: An update on clinical data.Front Oncol. 2022 Dec 22;12:1045481. doi: 10.3389/fonc.2022.1045481. eCollection 2022. Front Oncol. 2022. PMID: 36644634 Free PMC article. Review.
-
Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of gynecologic cancer.J Immunother Cancer. 2023 Jun;11(6):e006624. doi: 10.1136/jitc-2022-006624. J Immunother Cancer. 2023. PMID: 37295818 Free PMC article.
-
Viral vector-based therapeutic HPV vaccines.Clin Exp Med. 2024 Aug 28;24(1):199. doi: 10.1007/s10238-024-01470-5. Clin Exp Med. 2024. PMID: 39196444 Free PMC article. Review.
-
Human papilloma virus infection drives unique metabolic and immune profiles in head and neck and cervical cancers: implications for targeted therapies and prognostic markers.Discov Oncol. 2025 May 6;16(1):676. doi: 10.1007/s12672-025-02384-8. Discov Oncol. 2025. PMID: 40327200 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

