Comparison of non-myeloablative lymphodepleting preconditioning regimens in patients undergoing adoptive T cell therapy
- PMID: 33990415
- PMCID: PMC8127974
- DOI: 10.1136/jitc-2020-001743
Comparison of non-myeloablative lymphodepleting preconditioning regimens in patients undergoing adoptive T cell therapy
Abstract
Background: Adoptive cell therapy with T cells genetically engineered to express a chimeric antigen receptor (CAR-T) or tumor-infiltrating T lymphocytes (TIL) demonstrates impressive clinical results in patients with cancer. Lymphodepleting preconditioning prior to cell infusion is an integral part of all adoptive T cell therapies. However, to date, there is no standardization and no data comparing different non-myeloablative (NMA) regimens.
Methods: In this study, we compared NMA therapies with different doses of cyclophosphamide or total body irradiation (TBI) in combination with fludarabine and evaluated bone marrow suppression and recovery, cytokine serum levels, clinical response and adverse events.
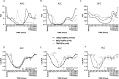
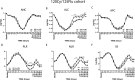
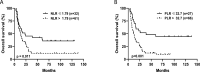
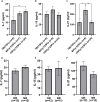
Results: We demonstrate that a cumulative dose of 120 mg/kg cyclophosphamide and 125 mg/m2 fludarabine (120Cy/125Flu) and 60Cy/125Flu preconditioning were equally efficient in achieving deep lymphopenia and neutropenia in patients with metastatic melanoma, whereas absolute lymphocyte counts (ALCs) and absolute neutrophil counts were significantly higher following 200 cGyTBI/75Flu-induced NMA. Thrombocytopenia was most profound in 120Cy/125Flu patients. 30Cy/75Flu-induced preconditioning in patients with acute lymphoblastic leukemia resulted in a minor ALC decrease, had no impact on platelet counts and did not yield deep neutropenia. Following cell infusion, 120Cy/125Flu patients with objective tumor response had significantly higher ALC and significant lower inflammatory indexes, such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR). Receiver-operating characteristics curve analysis 7 days after cell infusion was performed to determine the cut-offs, which distinguish between responding and non-responding patients in the 120Cy/125Flu cohort. NLR≤1.79 and PLR≤32.7 were associated with clinical response and overall survival. Cytokine serum levels did not associate with clinical response in patients with TIL. Patients in the 120Cy/125Flu cohort developed significantly more acute NMA-related adverse events, including thrombocytopenia, febrile neutropenia and cardiotoxicity, and stayed significantly longer in hospital compared with the 60Cy/125Flu and TBI/75Flu cohorts.
Conclusions: Bone marrow depletion and recovery were equally affected by 120Cy/125Flu and 60Cy/125Flu preconditioning; however, toxicity and consequently duration of hospitalization were significantly lower in the 60Cy/125Flu cohort. Patients in the 30Cy/75Flu and TBI/75Flu groups rarely developed NMA-induced adverse events; however, both regimens were not efficient in achieving deep bone marrow suppression. Among the regimens, 60Cy/125Flu preconditioning seems to achieve maximum effect with minimum toxicity.
Trial registration: ClinicalTrials.gov NCT00287131 NCT03166397 NCT02772198.
Keywords: adoptive; chimeric antigen; clinical trials; immunotherapy; lymphocytes; phase II as topic; receptors; tumor-infiltrating.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures
Similar articles
-
Randomized phase II trial of lymphodepletion plus adoptive cell transfer of tumor-infiltrating lymphocytes, with or without dendritic cell vaccination, in patients with metastatic melanoma.J Immunother Cancer. 2021 May;9(5):e002449. doi: 10.1136/jitc-2021-002449. J Immunother Cancer. 2021. PMID: 34021033 Free PMC article. Clinical Trial.
-
Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma.J Clin Oncol. 2005 Apr 1;23(10):2346-57. doi: 10.1200/JCO.2005.00.240. J Clin Oncol. 2005. PMID: 15800326 Free PMC article. Clinical Trial.
-
Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens.J Clin Oncol. 2008 Nov 10;26(32):5233-9. doi: 10.1200/JCO.2008.16.5449. Epub 2008 Sep 22. J Clin Oncol. 2008. PMID: 18809613 Free PMC article. Clinical Trial.
-
Is antigen specificity the key to efficient adoptive T-cell therapy?Immunotherapy. 2011 Apr;3(4):495-505. doi: 10.2217/imt.11.16. Immunotherapy. 2011. PMID: 21463191 Review.
-
Adoptive T-Cell Therapy in Melanoma: How This Will Impact Surgical Practice and the Role of Surgeons.Surg Oncol Clin N Am. 2025 Jul;34(3):423-436. doi: 10.1016/j.soc.2025.01.002. Epub 2025 Feb 27. Surg Oncol Clin N Am. 2025. PMID: 40413008 Review.
Cited by
-
Recent clinical researches and technological development in TIL therapy.Cancer Immunol Immunother. 2024 Sep 12;73(11):232. doi: 10.1007/s00262-024-03793-4. Cancer Immunol Immunother. 2024. PMID: 39264449 Free PMC article. Review.
-
Cardiovascular toxicities associated with novel cellular immune therapies.Blood Adv. 2024 Dec 24;8(24):6282-6296. doi: 10.1182/bloodadvances.2024013849. Blood Adv. 2024. PMID: 39418640 Free PMC article. Review.
-
Challenges and solutions for therapeutic TCR-based agents.Immunol Rev. 2023 Nov;320(1):58-82. doi: 10.1111/imr.13233. Epub 2023 Jul 16. Immunol Rev. 2023. PMID: 37455333 Free PMC article. Review.
-
Advances and prospects in tumor infiltrating lymphocyte therapy.Discov Oncol. 2024 Nov 8;15(1):630. doi: 10.1007/s12672-024-01410-5. Discov Oncol. 2024. PMID: 39514075 Free PMC article. Review.
-
Stem cell-derived CAR T cells show greater persistence, trafficking, and viral control compared to ex vivo transduced CAR T cells.Mol Ther. 2024 Apr 3;32(4):1000-1015. doi: 10.1016/j.ymthe.2024.02.026. Epub 2024 Feb 27. Mol Ther. 2024. PMID: 38414243 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
