Fluoroquinolone Antibiotics and Tendon Injury in Adolescents
- PMID: 33990459
- PMCID: PMC8168605
- DOI: 10.1542/peds.2020-033316
Fluoroquinolone Antibiotics and Tendon Injury in Adolescents
Abstract
Objectives: To estimate the association between fluoroquinolone use and tendon injury in adolescents.
Methods: We conducted an active-comparator, new-user cohort study using population-based claims data from 2000 to 2018. We included adolescents (aged 12-18 years) with an outpatient prescription fill for an oral fluoroquinolone or comparator broad-spectrum antibiotic. The primary outcome was Achilles, quadricep, patellar, or tibial tendon rupture identified by diagnosis and procedure codes. Tendinitis was a secondary outcome. We used weighting to adjust for measured confounding and a negative control outcome to assess residual confounding.
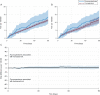
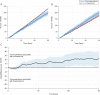
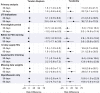
Results: The cohort included 4.4 million adolescents with 7.6 million fills for fluoroquinolone (275 767 fills) or comparator (7 365 684) antibiotics. In the 90 days after the index antibiotic prescription, there were 842 tendon ruptures and 16 750 tendinitis diagnoses (crude rates 0.47 and 9.34 per 1000 person-years, respectively). The weighted 90-day tendon rupture risks were 13.6 per 100 000 fluoroquinolone-treated adolescents and 11.6 per 100 000 comparator-treated adolescents (fluoroquinolone-associated excess risk: 1.9 per 100 000 adolescents; 95% confidence interval -2.6 to 6.4); the corresponding number needed to treat to harm was 52 632. For tendinitis, the weighted 90-day risks were 200.8 per 100 000 fluoroquinolone-treated adolescents and 178.1 per 100 000 comparator-treated adolescents (excess risk: 22.7 per 100 000; 95% confidence interval 4.1 to 41.3); the number needed to treat to harm was 4405.
Conclusions: The excess risk of tendon rupture associated with fluoroquinolone treatment was extremely small, and these events were rare. The excess risk of tendinitis associated with fluoroquinolone treatment was also small. Other more common potential adverse drug effects may be more important to consider for treatment decision-making, particularly in adolescents without other risk factors for tendon injury.
Copyright © 2021 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Herzog is an employee of IQVIA, a human data science company. Dr Jonsson Funk directs the Center for Pharmacoepidemiology and receives salary support through the Center for Pharmacoepidemiology from member companies, including GlaxoSmithKline, Merck (past member), Takeda, AbbVie, Boehringer Ingelheim, and UCB Bioscience, which have collaborative agreements with the Center for Pharmacoepidemiology, housed in the Department of Epidemiology. Dr Jonsson Funk is a member of the scientific steering committee for a postapproval safety study of an unrelated drug class funded by GlaxoSmithKline. All compensation for services provided on the scientific steering committee is invoiced by and paid to the University of North Carolina at Chapel Hill; and Ms Ross and Drs Kinlaw and Gerber have indicated they have no potential conflicts of interest to disclose.
Figures

Comment in
-
Perceived Harm May Be Helpful: Fear of Fluoroquinolone-Associated Adverse Events in Children.Pediatrics. 2021 Jun;147(6):e2021050321. doi: 10.1542/peds.2021-050321. Epub 2021 May 14. Pediatrics. 2021. PMID: 33990460 No abstract available.
References
-
- Elzagallaai AA, Greff M, Rieder MJ. Adverse drug reactions in children: the double-edged sword of therapeutics. Clin Pharmacol Ther. 2017;101(6):725–735 - PubMed
-
- US Food and Drug Administration. Drug research and children. 2016. Available at: https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm143565.htm. Accessed March 1, 2019
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

