Modulating the voltage sensor of a cardiac potassium channel shows antiarrhythmic effects
- PMID: 33990467
- PMCID: PMC8157969
- DOI: 10.1073/pnas.2024215118
Modulating the voltage sensor of a cardiac potassium channel shows antiarrhythmic effects
Abstract
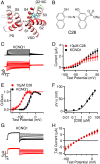
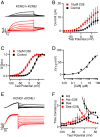
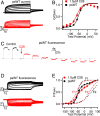
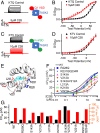
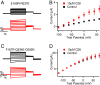
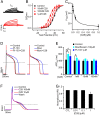
Cardiac arrhythmias are the most common cause of sudden cardiac death worldwide. Lengthening the ventricular action potential duration (APD), either congenitally or via pathologic or pharmacologic means, predisposes to a life-threatening ventricular arrhythmia, Torsade de Pointes. IKs (KCNQ1+KCNE1), a slowly activating K+ current, plays a role in action potential repolarization. In this study, we screened a chemical library in silico by docking compounds to the voltage-sensing domain (VSD) of the IKs channel. Here, we show that C28 specifically shifted IKs VSD activation in ventricle to more negative voltages and reversed the drug-induced lengthening of APD. At the same dosage, C28 did not cause significant changes of the normal APD in either ventricle or atrium. This study provides evidence in support of a computational prediction of IKs VSD activation as a potential therapeutic approach for all forms of APD prolongation. This outcome could expand the therapeutic efficacy of a myriad of currently approved drugs that may trigger arrhythmias.
Keywords: C28; IKs; KCNQ1; antiarrhythmia; voltage sensor domain.
Conflict of interest statement
Competing interest statement: J.S. and J.C. are cofounders of a startup company VivoCor LLC, which is targeting IKs for the treatment of cardiac arrhythmia.
Figures
References
-
- Keating M. T., Sanguinetti M. C., Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104, 569–580 (2001). - PubMed
-
- Wang H. S., Brown B. S., McKinnon D., Cohen I. S., Molecular basis for differential sensitivity of KCNQ and I(Ks) channels to the cognitive enhancer XE991. Mol. Pharmacol. 57, 1218–1223 (2000). - PubMed
-
- Barhanin J., et al., K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384, 78–80 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

