Comparison of gene expression and biotransformation activity of HepaRG cells under static and dynamic culture conditions
- PMID: 33990636
- PMCID: PMC8121841
- DOI: 10.1038/s41598-021-89710-6
Comparison of gene expression and biotransformation activity of HepaRG cells under static and dynamic culture conditions
Abstract
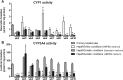
Flow conditions have been shown to be important in improving longevity and functionality of primary hepatocytes, but the impact of flow on HepaRG cells is largely unknown. We studied the expression of genes encoding CYP enzymes and transporter proteins and CYP1 and CYP3A4 activity during 8 weeks of culture in HepaRG cells cultured under static conditions (conventional 24-/96-well plate culture with common bicarbonate/CO2 buffering) and under flow conditions in an organ-on-chip (OOC) device. Since the OOC-device is a closed system, bicarbonate/CO2 buffering was not possible, requiring application of another buffering agent, such as HEPES. In order to disentangle the effects of HEPES from the effects of flow, we also applied HEPES-supplemented medium in static cultures and studied gene expression and CYP activity. We found that cells cultured under flow conditions in the OOC-device, as well as cells cultured under static conditions with HEPES-supplemented medium, showed more stable gene expression levels. Furthermore, only cells cultured in the OOC-device showed relatively high baseline CYP1 activity, and their gene expression levels of selected CYPs and transporters were most similar to gene expression levels in human primary hepatocytes. However, there was a decrease in baseline CYP3A4 activity under flow conditions compared to HepaRG cells cultured under static conditions. Altogether, the present study shows that HepaRG cells cultured in the OOC-device were more stable than in static cultures, being a promising in vitro model to study hepatoxicity of chemicals upon chronic exposure.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

