The loss of SHMT2 mediates 5-fluorouracil chemoresistance in colorectal cancer by upregulating autophagy
- PMID: 33990700
- PMCID: PMC8195740
- DOI: 10.1038/s41388-021-01815-4
The loss of SHMT2 mediates 5-fluorouracil chemoresistance in colorectal cancer by upregulating autophagy
Abstract
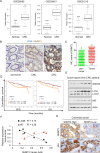
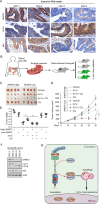
5-Fluorouracil (5-FU)-based chemotherapy is the first-line treatment for colorectal cancer (CRC) but is hampered by chemoresistance. Despite its impact on patient survival, the mechanism underlying chemoresistance against 5-FU remains poorly understood. Here, we identified serine hydroxymethyltransferase-2 (SHMT2) as a critical regulator of 5-FU chemoresistance in CRC. SHMT2 inhibits autophagy by binding cytosolic p53 instead of metabolism. SHMT2 prevents cytosolic p53 degradation by inhibiting the binding of p53 and HDM2. Under 5-FU treatment, SHMT2 depletion promotes autophagy and inhibits apoptosis. Autophagy inhibitors decrease low SHMT2-induced 5-FU resistance in vitro and in vivo. Finally, the lethality of 5-FU treatment to CRC cells was enhanced by treatment with the autophagy inhibitor chloroquine in patient-derived and CRC cell xenograft models. Taken together, our findings indicate that autophagy induced by low SHMT2 levels mediates 5-FU resistance in CRC. These results reveal the SHMT2-p53 interaction as a novel therapeutic target and provide a potential opportunity to reduce chemoresistance.
Conflict of interest statement
The authors declare no competing interests.
Figures
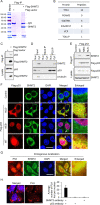
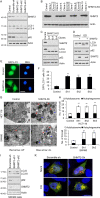

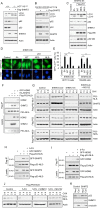
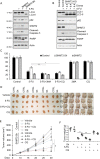
References
-
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–64. - PubMed
-
- Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1–10. - PubMed
-
- Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: a comprehensive review. Biomed Pharmacother. 2018;104:485–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

