Bispecific antibody simultaneously targeting PD1 and HER2 inhibits tumor growth via direct tumor cell killing in combination with PD1/PDL1 blockade and HER2 inhibition
- PMID: 33990766
- PMCID: PMC8888617
- DOI: 10.1038/s41401-021-00683-8
Bispecific antibody simultaneously targeting PD1 and HER2 inhibits tumor growth via direct tumor cell killing in combination with PD1/PDL1 blockade and HER2 inhibition
Abstract
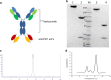
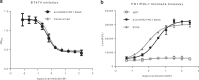
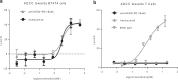
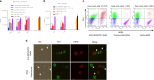
Immune checkpoint blockade has shown significant clinical benefit in multiple cancer indications, but many patients are either refractory or become resistant to the treatment over time. HER2/neu oncogene overexpressed in invasive breast cancer patients associates with more aggressive diseases and poor prognosis. Anti-HER2 mAbs, such as trastuzumab, are currently the standard of care for HER2-overexpressing cancers, but the response rates are below 30% and patients generally suffer relapse within a year. In this study we developed a bispecific antibody (BsAb) simultaneously targeting both PD1 and HER2 in an attempt to combine HER2-targeted therapy with immune checkpoint blockade for treating HER2-positive solid tumors. The BsAb was constructed by fusing scFvs (anti-PD1) with the effector-functional Fc of an IgG (trastuzumab) via a flexible peptide linker. We showed that the BsAb bound to human HER2 and PD1 with high affinities (EC50 values were 0.2 and 0.14 nM, respectively), and exhibited potent antitumor activities in vitro and in vivo. Furthermore, we demonstrated that the BsAb exhibited both HER2 and PD1 blockade activities and was effective in killing HER2-positive tumor cells via antibody-dependent cellular cytotoxicity. In addition, the BsAb could crosslink HER2-positive tumor cells with T cells to form PD1 immunological synapses that directed tumor cell killing without the need of antigen presentation. Thus, the BsAb is a new promising approach for treating late-stage metastatic HER2-positive cancers.
Keywords: HER2; PD1 blockade; PD1 immunological synapse; Trastuzumab; antibody-dependent cellular cytotoxicity (ADCC); bispecific antibody.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest. All authors are employees of Sunshine Guojian Pharmaceutical (Shanghai) when the study was carried out.
Figures

References
-
- Zhang X, Chen J, Weng Z, Li Q, Zhao L, Yu N, et al. A new anti-HER2 antibody that enhances the anti-tumor efficacy of trastuzumab and pertuzumab with a distinct mechanism of action. Mol Immunol. 2020;119:48–58. - PubMed
-
- Brandão M, Pondé NF, Poggio F, Kotecki N, Salis M, Lambertini M, et al. Combination therapies for the treatment of HER2-positive breast cancer: current and future prospects. Expert Rev Anticancer Ther. 2018;18:629–49. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

