Inborn disorders of the malate aspartate shuttle
- PMID: 33990986
- PMCID: PMC8362162
- DOI: 10.1002/jimd.12402
Inborn disorders of the malate aspartate shuttle
Abstract
Over the last few years, various inborn disorders have been reported in the malate aspartate shuttle (MAS). The MAS consists of four metabolic enzymes and two transporters, one of them having two isoforms that are expressed in different tissues. Together they form a biochemical pathway that shuttles electrons from the cytosol into mitochondria, as the inner mitochondrial membrane is impermeable to the electron carrier NADH. By shuttling NADH across the mitochondrial membrane in the form of a reduced metabolite (malate), the MAS plays an important role in mitochondrial respiration. In addition, the MAS maintains the cytosolic NAD+ /NADH redox balance, by using redox reactions for the transfer of electrons. This explains why the MAS is also important in sustaining cytosolic redox-dependent metabolic pathways, such as glycolysis and serine biosynthesis. The current review provides insights into the clinical and biochemical characteristics of MAS deficiencies. To date, five out of seven potential MAS deficiencies have been reported. Most of them present with a clinical phenotype of infantile epileptic encephalopathy. Although not specific, biochemical characteristics include high lactate, high glycerol 3-phosphate, a disturbed redox balance, TCA abnormalities, high ammonia, and low serine, which may be helpful in reaching a diagnosis in patients with an infantile epileptic encephalopathy. Current implications for treatment include a ketogenic diet, as well as serine and vitamin B6 supplementation.
Keywords: AGC1; AGC2; GOT2; MDH1; MDH2; NAD(H); inborn metabolic disorder; malate aspartate shuttle; redox.
© 2021 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
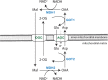
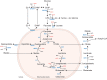
References
-
- Dawson AG. Oxidation of cytosolic NADH formed during aerobic metabolism in mammalian cells. Trends Biochem Sci. 1979;4(8):171‐176. 10.1016/0968-0004(79)90417-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

