Catalytic Generation of Carbanions through Carbonyl Umpolung
- PMID: 33991000
- PMCID: PMC8518712
- DOI: 10.1002/anie.202105469
Catalytic Generation of Carbanions through Carbonyl Umpolung
Abstract
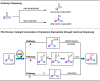
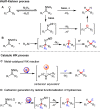
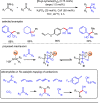
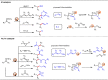
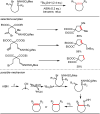
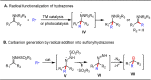
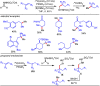
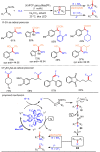
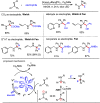
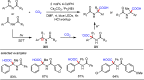
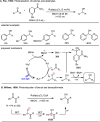
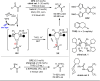
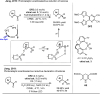
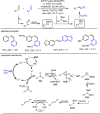
Carbonyl Umpolung is a powerful strategy in organic chemistry to construct complex molecules. Over the last few years, versatile catalytic approaches for the generation of acyl anion equivalents from carbonyl compounds have been developed, but methods to obtain alkyl carbanions from carbonyl compounds in a catalytic fashion are still at an early stage. This Minireview summarizes recent progress in the generation of alkyl carbanions through catalytic carbonyl Umpolung. Two different catalytic approaches can be utilized to enable the generation of alkyl carbanions from carbonyl compounds: the catalytic Wolff-Kishner reaction and the catalytic single-electron reduction of carbonyl compounds and imines. We discuss the reaction scope, mechanistic insights, and synthetic applications of the methods as well as potential future developments.
Keywords: Umpolung; Wolff-Kishner reaction; carbanions; photocatalysis.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
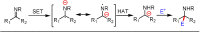

Similar articles
-
Carbonyl umpolung as an organometallic reagent surrogate.Chem Soc Rev. 2021 Oct 4;50(19):10733-10742. doi: 10.1039/d1cs00418b. Chem Soc Rev. 2021. PMID: 34382626 Review.
-
Umpolung Difunctionalization of Carbonyls via Visible-Light Photoredox Catalytic Radical-Carbanion Relay.J Am Chem Soc. 2020 Apr 22;142(16):7524-7531. doi: 10.1021/jacs.0c00629. Epub 2020 Apr 13. J Am Chem Soc. 2020. PMID: 32233431 Free PMC article.
-
Visible-light photoredox-catalyzed umpolung carboxylation of carbonyl compounds with CO2.Nat Commun. 2021 Jun 3;12(1):3306. doi: 10.1038/s41467-021-23447-8. Nat Commun. 2021. PMID: 34083530 Free PMC article.
-
Umpolung of Carbonyl Groups as Alkyl Organometallic Reagent Surrogates for Palladium-Catalyzed Allylic Alkylation.Angew Chem Int Ed Engl. 2018 Dec 10;57(50):16520-16524. doi: 10.1002/anie.201809112. Epub 2018 Nov 14. Angew Chem Int Ed Engl. 2018. PMID: 30351469
-
Catalytic asymmetric umpolung reactions of imines via 2-azaallyl anion intermediates.Org Biomol Chem. 2021 May 19;19(19):4193-4212. doi: 10.1039/d1ob00409c. Org Biomol Chem. 2021. PMID: 33870977 Review.
Cited by
-
Visible-light-driven alkene dicarboxylation with formate and CO2 under mild conditions.Chem Sci. 2024 Mar 21;15(16):6178-6183. doi: 10.1039/d3sc04431a. eCollection 2024 Apr 24. Chem Sci. 2024. PMID: 38665514 Free PMC article.
-
Photo-Induced Homologation of Carbonyl Compounds for Iterative Syntheses.Angew Chem Int Ed Engl. 2022 Dec 5;61(49):e202211578. doi: 10.1002/anie.202211578. Epub 2022 Nov 10. Angew Chem Int Ed Engl. 2022. PMID: 36226924 Free PMC article.
-
Nickel-Catalyzed Stereoselective Alkenylation of Ketones Mediated by Hydrazine.JACS Au. 2022 Jul 25;2(8):1929-1934. doi: 10.1021/jacsau.2c00320. eCollection 2022 Aug 22. JACS Au. 2022. PMID: 36032538 Free PMC article.
-
Electrochemically Driven Deoxygenative Borylation of Alcohols and Carbonyl Compounds.J Am Chem Soc. 2023 Aug 9;145(31):16966-16972. doi: 10.1021/jacs.3c03418. Epub 2023 Jul 27. J Am Chem Soc. 2023. PMID: 37499221 Free PMC article.
-
Enantioselective total syntheses of six natural and two proposed meroterpenoids from Psoralea corylifolia.Chem Sci. 2023 May 2;14(21):5699-5704. doi: 10.1039/d3sc00582h. eCollection 2023 May 31. Chem Sci. 2023. PMID: 37265714 Free PMC article.
References
-
- Wittig G., Davis P., Koenig G., Chem. Ber. 1951, 84, 627–632.
-
- Seebach D., Angew. Chem. Int. Ed. Engl. 1979, 18, 239–258;
- Angew. Chem. 1979, 91, 259–278.
-
- Wöhler F., Liebig J., Ann. Pharm. 1832, 3, 249–282.
-
- Corey E. J., Seebach D., Angew. Chem. Int. Ed. Engl. 1965, 4, 1075–1077;
- Angew. Chem. 1965, 77, 1134–1135.
-
- None
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

