(Automated) Synthesis of Well-defined Staphylococcus Aureus Wall Teichoic Acid Fragments
- PMID: 33991006
- PMCID: PMC8361686
- DOI: 10.1002/chem.202101242
(Automated) Synthesis of Well-defined Staphylococcus Aureus Wall Teichoic Acid Fragments
Abstract
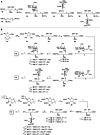
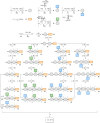
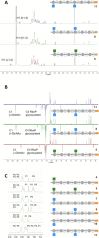
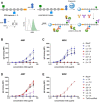
Wall teichoic acids (WTAs) are important components of the cell wall of the opportunistic Gram-positive bacterium Staphylococcus aureus. WTAs are composed of repeating ribitol phosphate (RboP) residues that are decorated with d-alanine and N-acetyl-d-glucosamine (GlcNAc) modifications, in a seemingly random manner. These WTA-modifications play an important role in shaping the interactions of WTA with the host immune system. Due to the structural heterogeneity of WTAs, it is impossible to isolate pure and well-defined WTA molecules from bacterial sources. Therefore, here synthetic chemistry to assemble a broad library of WTA-fragments, incorporating all possible glycosylation modifications (α-GlcNAc at the RboP C4; β-GlcNAc at the RboP C4; β-GlcNAc at the RboP C3) described for S. aureus WTAs, is reported. DNA-type chemistry, employing ribitol phosphoramidite building blocks, protected with a dimethoxy trityl group, was used to efficiently generate a library of WTA-hexamers. Automated solid phase syntheses were used to assemble a WTA-dodecamer and glycosylated WTA-hexamer. The synthetic fragments have been fully characterized and diagnostic signals were identified to discriminate the different glycosylation patterns. The different glycosylated WTA-fragments were used to probe binding of monoclonal antibodies using WTA-functionalized magnetic beads, revealing the binding specificity of these WTA-specific antibodies and the importance of the specific location of the GlcNAc modifications on the WTA-chains.
Keywords: antibodies; automated synthesis; gram-positive bacteria; ribitol phosphate; wall teichoic acids.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lowy F. D., N. Engl. J. Med. 1998, 339, 520. - PubMed
-
- None
-
- A. Fattom, (Nabi Biopharmaceuticals), WO 2007/053176 A2, 2007;
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

