Cost-effectiveness of multiparametric magnetic resonance imaging and MRI-guided biopsy in a population-based prostate cancer screening setting using a micro-simulation model
- PMID: 33991077
- PMCID: PMC8209626
- DOI: 10.1002/cam4.3932
Cost-effectiveness of multiparametric magnetic resonance imaging and MRI-guided biopsy in a population-based prostate cancer screening setting using a micro-simulation model
Abstract
Background: The introduction of multiparametric magnetic resonance imaging (mpMRI) and MRI-guided biopsy has improved the diagnosis of prostate cancer. However, it remains uncertain whether it is cost-effective, especially in a population-based screening strategy.
Methods: We used a micro-simulation model to assess the cost-effectiveness of an MRI-based prostate cancer screening in comparison to the classical prostate-specific antigen (PSA) screening, at a population level. The test sensitivity parameters for the mpMRI and MRI-guided biopsy, grade misclassification rates, utility estimates, and the unit costs of different interventions were obtained from literature. We assumed the same screening attendance rate and biopsy compliance rate for both strategies. A probabilistic sensitivity analysis, consisting of 1000 model runs, was performed to estimate a mean incremental cost-effectiveness ratio (ICER) and assess uncertainty. A €20,000 willingness-to-pay (WTP) threshold per quality-adjusted life year (QALY) gained, and a discounting rate of 3.5% was considered in the analysis.
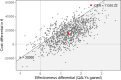
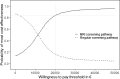
Results: The MRI-based screening improved the life-years (LY) and QALYs gained by 3.5 and 3, respectively, in comparison to the classical screening pathway. Based on the probabilistic sensitivity analyses, the MRI screening pathway leads to total discounted mean incremental costs of €15,413 (95% confidence interval (CI) of €14,556-€16,272) compared to the classical screening pathway. The corresponding discounted mean incremental QALYs gained was 1.36 (95% CI of 1.31-1.40), resulting in a mean ICER of €11,355 per QALY gained. At a WTP threshold of €20,000, the MRI screening pathway has about 84% chance to be more cost-effective than the classical screening pathway.
Conclusions: For triennial screening from age 55-64, incorporation of mpMRI as a reflex test after a positive PSA test result with a subsequent MRI-guided biopsy has a high probability to be more cost-effective as compared with the classical prostate cancer screening pathway.
Keywords: Cost-effectiveness analysis; MRI-guided biopsy; PSA Screening; mpMRI; prostate cancer.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures
References
-
- European . Policy paper on PSA screening for prostate cancer: Has the time come to reconsider structured population‐based PSA screening for prostate cancer? European Association of Urology. https://www.newsbook.com.mt/wp‐content/uploads/2019/04/EAU_policy‐briefi....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

