Serotonin and beyond-a tribute to Manfred Göthert (1939-2019)
- PMID: 33991216
- PMCID: PMC8376721
- DOI: 10.1007/s00210-021-02083-5
Serotonin and beyond-a tribute to Manfred Göthert (1939-2019)
Abstract
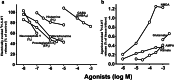
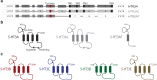
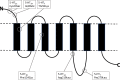
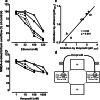
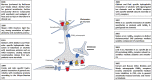
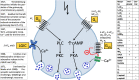
Manfred Göthert, who had served Naunyn-Schmiedeberg's Arch Pharmacol as Managing Editor from 1998 to 2005, deceased in June 2019. His scientific oeuvre encompasses more than 20 types of presynaptic receptors, mostly on serotoninergic and noradrenergic neurones. He was the first to identify presynaptic receptors for somatostatin and ACTH and described many presynaptic receptors, known from animal preparations, also in human tissue. In particular, he elucidated the pharmacology of presynaptic 5-HT receptors. A second field of interest included ligand-gated and voltage-dependent channels. The negative allosteric effect of anesthetics at peripheral nACh receptors is relevant for the peripheral clinical effects of these drugs and modified the Meyer-Overton hypothesis. The negative allosteric effect of ethanol at NMDA receptors in human brain tissue occurred at concentrations found in the range of clinical ethanol intoxication. Moreover, the inhibitory effect of gabapentinoids on P/Q Ca2+ channels and the subsequent decrease in AMPA-induced noradrenaline release may contribute to their clinical effect. Another ligand-gated ion channel, the 5-HT3 receptor, attracted the interest of Manfred Göthert from the whole animal via isolated preparations down to the cellular level. He contributed to that molecular study in which 5-HT3 receptor subtypes were disclosed. Finally, he found altered pharmacological properties of 5-HT receptor variants like the Arg219Leu 5-HT1A receptor (which was also shown to be associated with major depression) and the Phe124Cys 5-HT1B receptor (which may be related to sumatriptan-induced vasospasm). Manfred Göthert was a brilliant scientist and his papers have a major impact on today's pharmacology.
Keywords: 5-HT receptor mutants; 5-HT3 receptor structure and function; AMPA receptors; Mode of action of ethanol; Mode of action of anesthetics; Mode of action of gabapentinoids; NMDA receptors; Presynaptic receptors; Voltage-dependent cation channels; nACh receptors.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


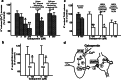

References
-
- Aktories K, Gierschik P, Meyer zu Heringdorf D, Schmidt M, Schultz G, Wieland T. cAMP guided his way: a life for G protein-mediated signal transduction and molecular pharmacology–tribute to Karl H. Jakobs. Naunyn Schmiedeberg's Arch Pharmacol. 2019;392:887–911. doi: 10.1007/s00210-019-01650-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Personal name as subject
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

