α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson's disease from multiple system atrophy
- PMID: 33991233
- PMCID: PMC8357708
- DOI: 10.1007/s00401-021-02324-0
α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson's disease from multiple system atrophy
Erratum in
-
Correction to: α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson's disease from multiple system atrophy.Acta Neuropathol. 2021 Sep;142(3):513. doi: 10.1007/s00401-021-02332-0. Acta Neuropathol. 2021. PMID: 34028589 Free PMC article. No abstract available.
Abstract
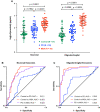
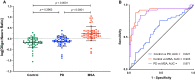
The diagnosis of Parkinson's disease (PD) and atypical parkinsonian syndromes is difficult due to the lack of reliable, easily accessible biomarkers. Multiple system atrophy (MSA) is a synucleinopathy whose symptoms often overlap with PD. Exosomes isolated from blood by immunoprecipitation using CNS markers provide a window into the brain's biochemistry and may assist in distinguishing between PD and MSA. Thus, we asked whether α-synuclein (α-syn) in such exosomes could distinguish among healthy individuals, patients with PD, and patients with MSA. We isolated exosomes from the serum or plasma of these three groups by immunoprecipitation using neuronal and oligodendroglial markers in two independent cohorts and measured α-syn in these exosomes using an electrochemiluminescence ELISA. In both cohorts, α-syn concentrations were significantly lower in the control group and significantly higher in the MSA group compared to the PD group. The ratio between α-syn concentrations in putative oligodendroglial exosomes compared to putative neuronal exosomes was a particularly sensitive biomarker for distinguishing between PD and MSA. Combining this ratio with the α-syn concentration itself and the total exosome concentration, a multinomial logistic model trained on the discovery cohort separated PD from MSA with an AUC = 0.902, corresponding to 89.8% sensitivity and 86.0% specificity when applied to the independent validation cohort. The data demonstrate that a minimally invasive blood test measuring α-syn in blood exosomes immunoprecipitated using CNS markers can distinguish between patients with PD and patients with MSA with high sensitivity and specificity. Future optimization and validation of the data by other groups would allow this strategy to become a viable diagnostic test for synucleinopathies.
Keywords: Biofluid; Biomarker; Extracellular vesicles; Synucleinopathy.
© 2021. The Author(s).
Conflict of interest statement
None of the authors has any financial disclosure or conflict of interest to report.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS082094/NS/NINDS NIH HHS/United States
- U54 NS065736/NS/NINDS NIH HHS/United States
- P30 AG072980/AG/NIA NIH HHS/United States
- R01 NS107596/NS/NINDS NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- K02 NS080915/NS/NINDS NIH HHS/United States
- R01 ES031106/ES/NIEHS NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- R01 ES010544/ES/NIEHS NIH HHS/United States
- U24 NS072026/NS/NINDS NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

