Photobiomodulation of oral fibroblasts stimulated with periodontal pathogens
- PMID: 33991267
- PMCID: PMC8593050
- DOI: 10.1007/s10103-021-03331-z
Photobiomodulation of oral fibroblasts stimulated with periodontal pathogens
Abstract
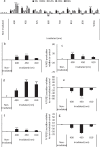
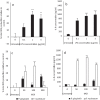
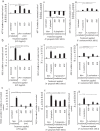
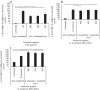
Photobiomodulation (PBM) utilises light energy to treat oral disease, periodontitis. However, there remains inconsistency in the reporting of treatment parameters and a lack of knowledge as to how PBM elicits its molecular effects in vitro. Therefore, this study aimed to establish the potential immunomodulatory effects of blue and near infra-red light irradiation on gingival fibroblasts (GFs), a key cell involved in the pathogenesis of periodontitis. GFs were seeded in 96-well plates in media + / - Escherichia coli lipopolysaccharide (LPS 1 μg/ml), or heat-killed Fusobacterium nucleatum (F. nucleatum, 100:1MOI) or Porphyromonas gingivalis (P. gingivalis, 500:1MOI). Cultures were incubated overnight and subsequently irradiated using a bespoke radiometrically calibrated LED array (400-830 nm, irradiance: 24 mW/cm2 dose: 5.76 J/cm2). Effects of PBM on mitochondrial activity (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and adenosine triphosphate (ATP) assays, total reactive oxygen species production (ROS assay) and pro-inflammatory/cytokine response (interleukin-8 (IL-8) and tumour growth factor-β1 (TGFβ1)) were assessed 24 h post-irradiation. Data were analysed using one-way ANOVA followed by the Tukey test. Irradiation of untreated (no inflammatory stimulus) cultures at 400 nm induced 15%, 27% and 13% increases in MTT, ROS and IL-8 levels, respectively (p < 0.05). Exposure with 450 nm light following application of P. gingivalis, F. nucleatum or LPS induced significant decreases in TGFβ1 secretion relative to their bacterially stimulated controls (p < 0.001). Following stimulation with P. gingivalis, 400 nm irradiation induced 14% increases in MTT, respectively, relative to bacteria-stimulated controls (p < 0.05). These findings could identify important irradiation parameters to enable management of the hyper-inflammatory response characteristic of periodontitis.
Keywords: Fibroblast; Mitochondria; PBM; Periodontitis; Photobiomodulation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Mester E, Szende B, Gärtner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother. 1968;9(5):621–626. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

