Pharmacokinetics and Comparative Bioavailability of Apomorphine Sublingual Film and Subcutaneous Apomorphine Formulations in Patients with Parkinson's Disease and "OFF" Episodes: Results of a Randomized, Three-Way Crossover, Open-Label Study
- PMID: 33991326
- PMCID: PMC8571442
- DOI: 10.1007/s40120-021-00251-6
Pharmacokinetics and Comparative Bioavailability of Apomorphine Sublingual Film and Subcutaneous Apomorphine Formulations in Patients with Parkinson's Disease and "OFF" Episodes: Results of a Randomized, Three-Way Crossover, Open-Label Study
Abstract
Introduction: In a pivotal study, apomorphine sublingual film (APL; KYNMOBI®) was an effective and generally well-tolerated on-demand treatment of "OFF" episodes in patients with Parkinson's disease (PD), approved across the dose range of 10-30 mg. Pharmacokinetics and comparative bioavailability of APL and two subcutaneous (SC) apomorphine formulations (SC-APO [APOKYN®] and SC-APO-GO [APO-go® PEN]) were evaluated in a randomized, three-way crossover, open-label study (NCT03292016).
Methods: Patients with PD and "OFF" episodes received an open-label randomized sequence of single doses of SC-APO and SC-APO-GO at the currently prescribed dose (2/3/4/5 mg) and APL doses with similar plasma exposure (15/20/25/30 mg) with ≥ 1-day washout between formulations. Plasma pharmacokinetics of apomorphine and apomorphine sulfate (major inactive metabolite) were measured 0-6 h postdose.
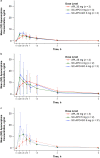
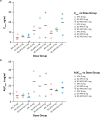
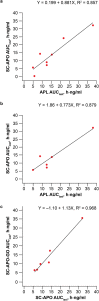
Results: Median time to maximum plasma concentration (tmax) of apomorphine was 0.63-0.75 h for APL and 0.25-0.38 h for SC-APO and SC-APO-GO. Geometric mean maximum plasma concentration (Cmax) of apomorphine was 4.31-11.2 ng/ml across APL doses and was generally lower compared with SC apomorphine formulations within dose groups. Area under the concentration-time curve from time 0 to infinity (AUC∞) was similar across apomorphine formulations within most dose groups. Relative bioavailability of APL was ~ 17% of SC apomorphine by AUC∞; SC-APO and SC-APO-GO had similar bioavailability (98% and 83% by AUC∞ and Cmax, respectively). Apomorphine sulfate exposure was ~ three-fold higher for APL versus SC-APO and SC-APO-GO by AUC∞ and Cmax.
Conclusion: In patients with PD and "OFF" episodes, APL demonstrated lower Cmax and relative bioavailability but similar exposures (AUCs) versus SC apomorphine within the approved dose range.
Trial registration: ClinicalTrials.gov, NCT03292016.
Keywords: APL; APL-130277; Apomorphine sublingual film; Bioavailability; Exposure; Parkinson’s disease; Pharmacokinetics; Subcutaneous apomorphine; “OFF” episodes.
© 2021. The Author(s).
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

