Population Pharmacokinetic Analysis of Bedaquiline-Clarithromycin for Dose Selection Against Pulmonary Nontuberculous Mycobacteria Based on a Phase 1, Randomized, Pharmacokinetic Study
- PMID: 33991350
- PMCID: PMC8518967
- DOI: 10.1002/jcph.1887
Population Pharmacokinetic Analysis of Bedaquiline-Clarithromycin for Dose Selection Against Pulmonary Nontuberculous Mycobacteria Based on a Phase 1, Randomized, Pharmacokinetic Study
Abstract
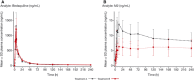
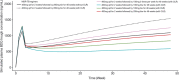
Based on the in vitro profile of bedaquiline against mycobacterial species, it is being investigated for clinical efficacy against pulmonary nontuberculous mycobacteria (PNTM). Being a cytochrome P450 3A substrate, pharmacokinetic interactions of bedaquiline are anticipated with clarithromycin (a cytochrome P450 3A inhibitor), which is routinely used in pulmonary nontuberculous mycobacteria treatment. This phase 1, randomized, crossover study assessed the impact of steady-state clarithromycin (500 mg every 12 hours for 14 days) on the pharmacokinetics of bedaquiline and its metabolite (M2) after single-dose bedaquiline (100 mg; n = 16). Using these data, population pharmacokinetic modeling and simulation analyses were performed to determine the effect of clarithromycin on steady-state bedaquiline exposure. Although no effect was observed on maximum plasma concentration of bedaquiline and time to achieve maximum plasma concentration, its mean plasma exposure increased by 14% after 10 days of clarithromycin coadministration, with slower formation of M2. Simulations showed that bedaquiline plasma trough concentration at steady state was higher (up to 41% until week 48) with clarithromycin coadministration as compared to its monotherapy (400 mg once daily for 2 weeks, followed by 200 mg 3 times a week for 46 weeks; reference regimen). The overall exposure of a simulated bedaquiline regimen (400 mg once dialy for 2 weeks, followed by 200 mg twice a week for 46 weeks) with clarithromycin was comparable (<15% difference) to the monotherapy. Overall, combination of bedaquiline (400 mg once daily for 2 weeks, followed by 200 mg twice a week for 46 weeks) with clarithromycin seems a suitable regimen to be explored for efficacy and safety against pulmonary nontuberculous mycobacteria.
Keywords: CYP3A inhibitors; bedaquiline; clarithromycin; drug interaction; pharmacokinetics; pulmonary nontuberculous mycobacteria disease.
© 2021 Janssen Research and Development, Beerse, Belgium. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
All authors are employees of Janssen Pharmaceutical Companies of Johnson and Johnson. Further, Ken Kurosawa, Stefaan Rossenu, Sivi Ouwerkerk‐Mahadevan, Wouter Willems, and Chrispin Kambili are potential stockholders of Johnson and Johnson.
Figures

Similar articles
-
In Vitro Activity of Bedaquiline and Delamanid against Nontuberculous Mycobacteria, Including Macrolide-Resistant Clinical Isolates.Antimicrob Agents Chemother. 2019 Jul 25;63(8):e00665-19. doi: 10.1128/AAC.00665-19. Print 2019 Aug. Antimicrob Agents Chemother. 2019. PMID: 31182533 Free PMC article.
-
In Vitro Activity of Bedaquiline against Nontuberculous Mycobacteria in China.Antimicrob Agents Chemother. 2017 Apr 24;61(5):e02627-16. doi: 10.1128/AAC.02627-16. Print 2017 May. Antimicrob Agents Chemother. 2017. PMID: 28242674 Free PMC article.
-
In Vitro Activities of Bedaquiline and Delamanid against Nontuberculous Mycobacteria Isolated in Beijing, China.Antimicrob Agents Chemother. 2019 Jul 25;63(8):e00031-19. doi: 10.1128/AAC.00031-19. Print 2019 Aug. Antimicrob Agents Chemother. 2019. PMID: 31138571 Free PMC article.
-
Bedaquiline: a novel antitubercular agent for the treatment of multidrug-resistant tuberculosis.Pharmacotherapy. 2014 Nov;34(11):1187-97. doi: 10.1002/phar.1482. Epub 2014 Sep 9. Pharmacotherapy. 2014. PMID: 25203970 Review.
-
Bedaquiline as part of combination therapy in adults with pulmonary multi-drug resistant tuberculosis.Expert Rev Clin Pharmacol. 2016 Aug;9(8):1025-37. doi: 10.1080/17512433.2016.1200462. Epub 2016 Jun 27. Expert Rev Clin Pharmacol. 2016. PMID: 27322153 Review.
Cited by
-
Infant Exposure to Antituberculosis Drugs via Breast Milk and Assessment of Potential Adverse Effects in Breastfed Infants: Critical Review of Data.Pharmaceutics. 2023 Apr 13;15(4):1228. doi: 10.3390/pharmaceutics15041228. Pharmaceutics. 2023. PMID: 37111713 Free PMC article. Review.
-
Population pharmacokinetics of bedaquiline: a systematic review.Eur J Clin Pharmacol. 2025 Mar;81(3):347-363. doi: 10.1007/s00228-024-03788-1. Epub 2025 Jan 8. Eur J Clin Pharmacol. 2025. PMID: 39779577
References
-
- Haworth CS, Banks J, Capstick T, et al. British Thoracic Society guidelines for the management of non‐tuberculous mycobacterial pulmonary disease (NTM‐PD). Thorax. 2017;72(suppl 2):ii1‐ii64. - PubMed
-
- Griffith DE, Aksamit T, Brown‐Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction appears in Am J Respir Crit Care Med. 2007;175(7):744‐745. Dosage error in article text]. Am J Respir Crit Care Med. 2007;175(4):367‐416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

