Coronavirus disease 2019 (COVID-19) vaccines: A concise review
- PMID: 33991381
- PMCID: PMC8242875
- DOI: 10.1111/odi.13916
Coronavirus disease 2019 (COVID-19) vaccines: A concise review
Abstract
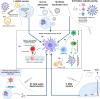
The development of a successful vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the agent of coronavirus disease 2019 (COVID-19), in an unmatched period of ten months, is a tribute to human ingenuity in the face of a vicious pandemic. A return to pre-pandemic "normalcy" depends on the successful delivery of the vaccine to a majority (~70%) so as to develop herd immunity critical to arrest the community spread of infection. Vaccination against COVID-19 is particularly important for dentistry as the dental team works in an environment replete with aerosol-generating procedures (AGP) that facilitate virus spread. Hence, a COVID-19 vaccine is likely to be an obligatory requirement for the dental practice, and the latest addition to the extensive list of vaccines required for dental professionals for the safe delivery of dental care. Here, we review the currently available major candidate vaccines against SARS-CoV-2 and their benefits and risks. These include the vaccines developed on next-generation platforms (mRNA, DNA, and viral vector vaccines), and the classic platforms (the live-attenuated virus, and the protein subunit vaccines) The review concludes with a summary of impending issues and challenges facing the provision of COVID-19 vaccines for all stakeholders in dentistry.
Keywords: challenges; coronavirus disease 2019; impact; platforms; severe acute respiratory syndrome coronavirus 2; vaccines.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- CDC (2020). Summary of infection prevention practices in dental settings. Centers for Disease Control and Prevention (CDC), https://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe‐care2.pdf, 1‐44.
-
- CDC (2021a). About COVID‐19 Vaccination. Centers for Disease Control and Prevention. https://www.CDC.gov/coronavirus/2019‐ncov/vaccines/faq.html. 1‐3
-
- CDC . (2021b). Emerging SARS‐CoV‐2 Variants. Centers for Disease Control and Prevention (CDC). https://www.CDC.gov/coronavirus/2019‐ncov/more/science‐and‐research/scie.... 1‐3 - PubMed
-
- CDC . (2021c). How to protect yourself and others. Centers for Disease Control and Prevention, March 8 2021. 1‐2.
-
- Clementini, M. , Raspini, M. , Barbato, L. , Bernardelli, F. , Braga, G. , Di Gioia, C. , Littarru, C. , Oreglia, F. , Brambilla, E. , Iavicoli, I. , Pinchi, V. , Landi, L. , Marco Sforza, N. , Cavalcanti, R. , Crea, A. , & Cairo, F. . (2020). Aerosol transmission for SARS‐CoV‐2 in the dental practice. A review by SIdP Covid‐19 task‐force. Oral Diseases. 10.1111/odi.13649 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

