Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial
- PMID: 33991479
- PMCID: PMC8192829
- DOI: 10.1016/S0140-6736(21)00731-5
Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial
Abstract
Background: Ovarian cancer continues to have a poor prognosis with the majority of women diagnosed with advanced disease. Therefore, we undertook the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) to determine if population screening can reduce deaths due to the disease. We report on ovarian cancer mortality after long-term follow-up in UKCTOCS.
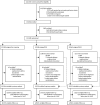
Methods: In this randomised controlled trial, postmenopausal women aged 50-74 years were recruited from 13 centres in National Health Service trusts in England, Wales, and Northern Ireland. Exclusion criteria were bilateral oophorectomy, previous ovarian or active non-ovarian malignancy, or increased familial ovarian cancer risk. The trial management system confirmed eligibility and randomly allocated participants in blocks of 32 using computer generated random numbers to annual multimodal screening (MMS), annual transvaginal ultrasound screening (USS), or no screening, in a 1:1:2 ratio. Follow-up was through national registries. The primary outcome was death due to ovarian or tubal cancer (WHO 2014 criteria) by June 30, 2020. Analyses were by intention to screen, comparing MMS and USS separately with no screening using the versatile test. Investigators and participants were aware of screening type, whereas the outcomes review committee were masked to randomisation group. This study is registered with ISRCTN, 22488978, and ClinicalTrials.gov, NCT00058032.
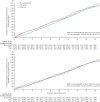
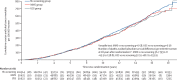
Findings: Between April 17, 2001, and Sept 29, 2005, of 1 243 282 women invited, 202 638 were recruited and randomly assigned, and 202 562 were included in the analysis: 50 625 (25·0%) in the MMS group, 50 623 (25·0%) in the USS group, and 101 314 (50·0%) in the no screening group. At a median follow-up of 16·3 years (IQR 15·1-17·3), 2055 women were diagnosed with tubal or ovarian cancer: 522 (1·0%) of 50 625 in the MMS group, 517 (1·0%) of 50 623 in the USS group, and 1016 (1·0%) of 101 314 in the no screening group. Compared with no screening, there was a 47·2% (95% CI 19·7 to 81·1) increase in stage I and 24·5% (-41·8 to -2·0) decrease in stage IV disease incidence in the MMS group. Overall the incidence of stage I or II disease was 39·2% (95% CI 16·1 to 66·9) higher in the MMS group than in the no screening group, whereas the incidence of stage III or IV disease was 10·2% (-21·3 to 2·4) lower. 1206 women died of the disease: 296 (0·6%) of 50 625 in the MMS group, 291 (0·6%) of 50 623 in the USS group, and 619 (0·6%) of 101 314 in the no screening group. No significant reduction in ovarian and tubal cancer deaths was observed in the MMS (p=0·58) or USS (p=0·36) groups compared with the no screening group.
Interpretation: The reduction in stage III or IV disease incidence in the MMS group was not sufficient to translate into lives saved, illustrating the importance of specifying cancer mortality as the primary outcome in screening trials. Given that screening did not significantly reduce ovarian and tubal cancer deaths, general population screening cannot be recommended.
Funding: National Institute for Health Research, Cancer Research UK, and The Eve Appeal.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests UM has stock ownership awarded by University College London (UCL) in Abcodia, which holds the licence for ROCA. She has received grants from the Medical Research Council (MRC), Cancer Research UK, the National Institute for Health Research (NIHR), and The Eve Appeal. She holds patent number EP10178345.4 for Breast Cancer Diagnostics. MP has received grants and AG-M, MB, AR, CK, GC, and SKM have been funded by grants from MRC, Cancer Research UK, NIHR, and The Eve Appeal. RM has received grants from The Eve Appeal, Rosetrees Charity, and Barts Charity, and personal fees from AstraZeneca. SJS holds the (expired) patent for ROCA, patented and owned by Massachusetts General Hospital and Queen Mary University of London, licenced to Abcodia. He reports stock options from SISCAPA Assay Technologies, and personal fees from Abcodia, Guardant Health, and Freenome, outside the submitted work. IJJ reports grants from Eve Appeal Charity, Medical Research Council, Cancer Research UK, and NIHR during the conduct of the study. He co-invented the ROCA in 1995, it was patented by Massachusetts General Hospital and Queen Mary University of London and is owned by these universities. Massachusetts General Hospital and Queen Mary University of London granted a licence to ROCA to Abcodia in 2014. IJJ is non-executive director, shareholder, and consultant to Abcodia and has rights to royalties from sales of the ROCA. He founded (1985), was a trustee of (2012–14), and is now an Emeritus trustee (2015–present) of The Eve Appeal, one of the funding agencies for UKCTOCS. All other authors declare no competing interests.
Figures
Comment in
-
General population screening for ovarian cancer.Lancet. 2021 Jun 5;397(10290):2128-2130. doi: 10.1016/S0140-6736(21)01061-8. Epub 2021 May 12. Lancet. 2021. PMID: 33991478 No abstract available.
-
In postmenopausal women, multimodal or US screening for ovarian cancer did not reduce ovarian cancer mortality.Ann Intern Med. 2021 Oct;174(10):JC114. doi: 10.7326/ACPJ202110190-114. Epub 2021 Oct 5. Ann Intern Med. 2021. PMID: 34606322
References
-
- Cancer Research UK Ovarian cancer survival statistics. 2018. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s...
-
- Jacobs IJ, Skates SJ, MacDonald N. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353:1207–1210. - PubMed
-
- Kobayashi H, Yamada Y, Sado T. A randomized study of screening for ovarian cancer: a multicenter study in Japan. Int J Gynecol Cancer. 2008;18:414–420. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

