Non-invasive, neurotoxic surgery reduces seizures in a rat model of temporal lobe epilepsy
- PMID: 33991523
- PMCID: PMC8937852
- DOI: 10.1016/j.expneurol.2021.113761
Non-invasive, neurotoxic surgery reduces seizures in a rat model of temporal lobe epilepsy
Abstract
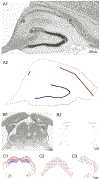
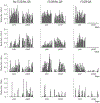
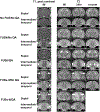
Surgery can be highly effective for treating certain cases of drug resistant epilepsy. The current study tested a novel, non-invasive, surgical strategy for treating seizures in a rat model of temporal lobe epilepsy. The surgical approach uses magnetic resonance-guided, low-intensity focused ultrasound (MRgFUS) in combination with intravenous microbubbles to open the blood-brain barrier (BBB) in a transient and focal manner. During the period of BBB opening, a systemically administered neurotoxin (Quinolinic Acid: QA) that is normally impermeable to the BBB gains access to a targeted area in the brain, destroying neurons where the BBB has been opened. This strategy is termed Precise Intracerebral Non-invasive Guided Surgery (PING). Spontaneous recurrent seizures induced by pilocarpine were monitored behaviorally prior to and after PING or under control conditions. Seizure frequency in untreated animals or animals treated with MRgFUS without QA exhibited expected seizure rate fluctuations frequencies between the monitoring periods. In contrast, animals treated with PING targeting the intermediate-temporal aspect of the hippocampus exhibited substantial reductions in seizure frequency, with convulsive seizures being eliminated entirely in two animals. These findings suggest that PING could provide a useful alternative to invasive surgical interventions for treating drug resistant epilepsy, and perhaps for treating other neurological disorders in which aberrant neural circuitries play a role.
Keywords: Epilepsy surgery; Focused ultrasound; Magnetic resonance-guided; Neuronal loss; Non-invasive; Quinolinic acid.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
These are no financial and non-financial competing interests.
Figures
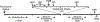
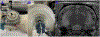
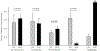


References
-
- Arida RM, Scorza FA, Peres CA, Cavalheiro EA, 1999. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res 34, 99–107. - PubMed
-
- Baud MO, Perneger T, Racz A, Pensel MC, Elger C, Rydenhag B, Malmgren K, Cross JH, McKenna G, Tisdall M, Lamberink HJ, Rheims S, Ryvlin P, Isnard J, Mauguiere F, Arzimanoglou A, Akkol S, Deniz K, Ozkara C, Lossius M, Rektor I, Kalviainen R, Vanhatalo LM, Dimova P, Minkin K, Staack AM, Steinhoff BJ, Kalina A, Krsek P, Marusic P, Jordan Z, Fabo D, Carrette E, Boon P, Rocka S, Mameniskiene R, Vulliemoz S, Pittau F, Braun KPJ, Seeck M, 2018b. European trends in epilepsy surgery. Neurology 91, e96–e106. - PubMed
-
- Baud MO, Ghestem A, Benoliel JJ, Becker C, Bernard C, 2019. Endogenous multidien rhythm of epilepsy in rats. Exp. Neurol 315, 82–87. - PubMed
-
- Beskid M, Rozycka Z, Taraszewska A, 1997. Quinolinic acid: effect on the nucleus arcuatus of the hypothalamus in the rat (ultrastructural evidence). Exp. Toxicol. Pathol 49, 477–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

