Cervical spinal cord injury-induced neuropathic pain in male mice is associated with a persistent pro-inflammatory macrophage/microglial response in the superficial dorsal horn
- PMID: 33991526
- PMCID: PMC8286318
- DOI: 10.1016/j.expneurol.2021.113757
Cervical spinal cord injury-induced neuropathic pain in male mice is associated with a persistent pro-inflammatory macrophage/microglial response in the superficial dorsal horn
Abstract
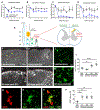
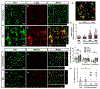
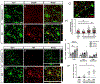
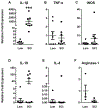
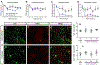
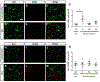
A significant portion of individuals living with traumatic spinal cord injury (SCI) experiences some degree of debilitating neuropathic pain (NP). This pain remains largely intractable in a majority of cases, due in part to an incomplete understanding of its underlying mechanisms. Central sensitization, an increase in excitability of pain transmission neurons located in superficial dorsal horn (sDH), plays a key role in development and maintenance of SCI-induced NP. Resident microglia and peripheral monocyte-derived macrophages (referred to collectively as MMΦ) are involved in promoting SCI-induced DH neuron hyperexcitability. Importantly, these MMΦ consist of populations of cells that can exert pro-inflammatory or anti-inflammatory signaling within injured spinal cord. It is critical to spatiotemporally characterize this heterogeneity to understand MMΦ contribution to NP after SCI. Given that a majority of SCI cases are cervical in nature, we used a model of unilateral C5/C6 contusion that results in persistent at-level thermal hyperalgesia and mechanical allodynia, two forms of NP-related behavior, in the forepaw. The aim of this study was to characterize the sDH MMΦ response within intact cervical spinal cord segments caudal to the lesion (i.e. the location of primary afferent nociceptive input from the forepaw plantar surface). Cervical SCI promoted a persistent MMΦ response in sDH that coincided with the chronic NP phenotype. Using markers of pro- and anti-inflammatory MMΦ, we found that the MMΦ population within sDH exhibited significant heterogeneity that evolved over time post-injury, including a robust and persistent increase in pro-inflammatory MMΦ that was especially pronounced at later times. C5/C6 contusion SCI also induced below-level thermal hyperalgesia and mechanical allodynia in the hindpaw; however, we did not observe a pronounced MMΦ response in sDH of L4/L5 spinal cord, suggesting that different inflammatory cell mechanisms occurring in sDH may be involved in at-level versus below-level NP following SCI. In conclusion, our findings reveal significant MMΦ heterogeneity both within and across pain transmission locations after SCI. These data also show a prominent and persistent pro-inflammatory MMΦ response, suggesting a possible role in DH neuron hyperexcitability and NP.
Keywords: Central sensitization; Contusion; Dorsal horn; Heterogeneity; Hyperexcitability; Macrophage; Neuropathic pain; SCI; Spinal cord injury.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- NB Finnerap IJ, sindrup SH Bach FW, Jensen TS, Pain and Dysesthesia in Patients with Spinal Cord Injury a Postal Survey. Spinal Cord, 2001. 39: p. 256–262. - PubMed
-
- Siddall PJ, et al., A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain, 2003. 103(3): p. 249–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

