Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy
- PMID: 33991637
- PMCID: PMC8285256
- DOI: 10.1016/j.jhep.2021.04.050
Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy
Abstract
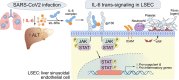
Background and aims: COVID-19 is associated with liver injury and elevated interleukin-6 (IL-6). We hypothesized that IL-6 trans-signaling in liver sinusoidal endothelial cells (LSECs) leads to endotheliopathy (a proinflammatory and procoagulant state) and liver injury in COVID-19.
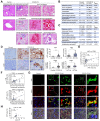
Methods: Coagulopathy, endotheliopathy, and alanine aminotransferase (ALT) were retrospectively analyzed in a subset (n = 68), followed by a larger cohort (n = 3,780) of patients with COVID-19. Liver histology from 43 patients with COVID-19 was analyzed for endotheliopathy and its relationship to liver injury. Primary human LSECs were used to establish the IL-6 trans-signaling mechanism.
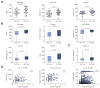
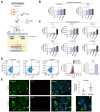
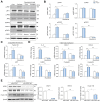
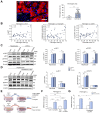
Results: Factor VIII, fibrinogen, D-dimer, von Willebrand factor (vWF) activity/antigen (biomarkers of coagulopathy/endotheliopathy) were significantly elevated in patients with COVID-19 and liver injury (elevated ALT). IL-6 positively correlated with vWF antigen (p = 0.02), factor VIII activity (p = 0.02), and D-dimer (p <0.0001). On liver histology, patients with COVID-19 and elevated ALT had significantly increased vWF and platelet staining, supporting a link between liver injury, coagulopathy, and endotheliopathy. Intralobular neutrophils positively correlated with platelet (p <0.0001) and vWF (p <0.01) staining, and IL-6 levels positively correlated with vWF staining (p <0.01). IL-6 trans-signaling leads to increased expression of procoagulant (factor VIII, vWF) and proinflammatory factors, increased cell surface vWF (p <0.01), and increased platelet attachment in LSECs. These effects were blocked by soluble glycoprotein 130 (IL-6 trans-signaling inhibitor), the JAK inhibitor ruxolitinib, and STAT1/3 small-interfering RNA knockdown. Hepatocyte fibrinogen expression was increased by the supernatant of LSECs subjected to IL-6 trans-signaling.
Conclusion: IL-6 trans-signaling drives the coagulopathy and hepatic endotheliopathy associated with COVID-19 and could be a possible mechanism behind liver injury in these patients.
Lay summary: Patients with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infection often have liver injury, but why this occurs remains unknown. High levels of interleukin-6 (IL-6) and its circulating receptor, which form a complex to induce inflammatory signals, have been observed in patients with COVID-19. This paper demonstrates that the IL-6 signaling complex causes harmful changes to liver sinusoidal endothelial cells and may promote blood clotting and contribute to liver injury.
Keywords: SARS-CoV-2; coagulopathy; endothelial cell dysfunction; thrombosis.
Copyright © 2021 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Comment in
-
Unraveling the role of liver sinusoidal endothelial cells in COVID-19 liver injury.J Hepatol. 2021 Sep;75(3):503-505. doi: 10.1016/j.jhep.2021.07.008. Epub 2021 Jul 15. J Hepatol. 2021. PMID: 34274367 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

