Universal Engraftment after Allogeneic Hematopoietic Cell Transplantation Using Cryopreserved CD34-Selected Grafts
- PMID: 33991721
- PMCID: PMC8316317
- DOI: 10.1016/j.jtct.2021.04.026
Universal Engraftment after Allogeneic Hematopoietic Cell Transplantation Using Cryopreserved CD34-Selected Grafts
Abstract
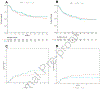
As a result of the COVID-19 pandemic, most centers performing allogeneic hematopoietic cell transplantation (allo-HCT) have switched to the use of cryopreserved grafts. Previous investigators have suggested that cryopreserved allografts may heighten risk of nonengraftment. To date, no study has investigated the effect of cryopreservation of CD34-selected hematopoietic progenitor cells (CD34+ HPCs) used as the sole graft source. In this study, we sought to evaluate outcomes after unrelated donor or matched sibling allo-HCT with cryopreserved CD34+ HPCs. This was a single-center analysis of adult patients with hematologic malignancies who underwent allo-HCT with cryopreserved CD34-selected allo-HCT grafts between January 2010 and June 2017. All patients received ablative conditioning and antirejection prophylaxis with rabbit antithymocyte globulin. G-CSF-mobilized leukapheresis products underwent CD34 selection using the CliniMACS Reagent System. Cells were then cryopreserved in DMSO (final concentration 7.5%) to -90 °C using a controlled-rate freezing system before being transferred to vapor-phase liquid nitrogen storage. In internal validation, this method has shown 92% mean CD34+ cell viability and 99.7% mean CD34+ cell recovery. Engraftment was defined as the first of 3 consecutive days of an absolute neutrophil count of ≥0.5. Platelet recovery was recorded as the first of 7 consecutive days with a platelet count ≥20 K/μL without transfusion. Kaplan-Meier methodology was used to estimate overall survival (OS) and relapse-free survival (RFS), and cumulative incidence functions were used to estimate rates of relapse, nonrelapse mortality (NRM), and acute graft-versus-host disease (GVHD). A total of 64 patients received a cryopreserved CD34-selected graft. The median CD34+ cell count before cryopreservation was 6.6 × 106/kg (range, 1.4 to 16.1 × 106/kg), and the median CD3+ cell count was 2.0 × 103/kg (range, 0 to 21.1 × 106/kg). All patients were engrafted, at a median of 11 days post-HCT (range, 8 to 14 days). One patient had poor graft function in the setting of cytomegalovirus viremia, necessitating a CD34-selected boost on day +57. The median time to platelet recovery was 16 days (range, 13 to 99 days). The estimated 2-year OS was 70% (95% confidence interval [CI], 58% to 83%) with cryopreserved grafts versus 62% (95% CI, 57% to 67%) with fresh grafts (hazard ratio [HR], 0.86; 95% CI, 0.54 to 1.35; P = .5). The estimated 2-year RFS in the 2 groups was 59% (95% CI, 48% to 74%) versus 56% (95% CI, 51% to 61%; HR, 1.01; 95% CI, 0.68 to 1.51; P > .9). The cumulative incidence of relapse at 2 years was 29% (95% CI, 17% to 41%) versus 23% (95% CI, 19% to 27%; P = .16), and the cumulative incidence of NRM at 2 years was 17% (95% CI, 9% to 28%) versus 23% (95% CI, 19% to 28%; P = .24). The cumulative incidence of grade II-IV acute GVHD by day +100 was 16% with cryopreserved grafts (95% CI, 8% to 26%) and 16% (95% CI, 13% to 20%; P = .97) with fresh grafts. Moderate to severe chronic GVHD by day +365 occurred in only 1 recipient of a cryopreserved graft (2%). Our data show that in patients with hematologic malignancies who received cryopreserved allogeneic CD34+ HPCs, engraftment, GVHD, and survival outcomes were consistent with those seen in recipients of fresh allogeneic CD34+ HPC grafts at our center. Our laboratory validation and clinical experience demonstrate the safety of our cryopreservation procedure for CD34-selected allografts.
Keywords: Allogeneic hematopoietic stem cell transplantation; CD34 selection; COVID-19; Cryopreservation; Engraftment.
Copyright © 2021 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
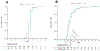
Similar articles
-
Graft Cryopreservation Does Not Impact Overall Survival after Allogeneic Hematopoietic Cell Transplantation Using Post-Transplantation Cyclophosphamide for Graft-versus-Host Disease Prophylaxis.Biol Blood Marrow Transplant. 2020 Jul;26(7):1312-1317. doi: 10.1016/j.bbmt.2020.04.001. Epub 2020 Apr 10. Biol Blood Marrow Transplant. 2020. PMID: 32283185 Free PMC article.
-
Outcomes with allogeneic stem cell transplant using cryopreserved versus fresh hematopoietic progenitor cell products.Cytotherapy. 2024 Oct;26(10):1210-1216. doi: 10.1016/j.jcyt.2024.05.009. Epub 2024 May 8. Cytotherapy. 2024. PMID: 38819367
-
Real-World Experience of Cryopreserved Allogeneic Hematopoietic Grafts during the COVID-19 Pandemic: A Single-Center Report.Transplant Cell Ther. 2022 Apr;28(4):215.e1-215.e10. doi: 10.1016/j.jtct.2022.01.010. Epub 2022 Jan 15. Transplant Cell Ther. 2022. PMID: 35042013 Free PMC article.
-
Outcomes with CD34-Selected Stem Cell Boost for Poor Graft Function after Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis.Transplant Cell Ther. 2021 Oct;27(10):877.e1-877.e8. doi: 10.1016/j.jtct.2021.07.012. Epub 2021 Jul 18. Transplant Cell Ther. 2021. PMID: 34284148
-
The effect of cryopreservation on engraftment kinetics in fully matched allogeneic stem cell transplantation: Real-life data and literature review.Transfus Apher Sci. 2023 Dec;62(6):103821. doi: 10.1016/j.transci.2023.103821. Epub 2023 Sep 18. Transfus Apher Sci. 2023. PMID: 37775358 Review.
Cited by
-
Secondary Impact of the Coronavirus Disease 19 Pandemic on Patients and the Cellular Therapy Healthcare Ecosystem.Transplant Cell Ther. 2022 Nov;28(11):737-746. doi: 10.1016/j.jtct.2022.07.020. Epub 2022 Jul 25. Transplant Cell Ther. 2022. PMID: 35902050 Free PMC article. Review.
-
Impact of Cryopreservation of Peripheral Blood Stem Cells (PBSC) in Transplantation from Matched Unrelated Donor (MUD).J Clin Med. 2022 Jul 15;11(14):4114. doi: 10.3390/jcm11144114. J Clin Med. 2022. PMID: 35887878 Free PMC article.
-
Pattern of Immune Reconstitution Post Allogeneic Stem Cell Transplant: Data From a Resource Constraint Country.Cureus. 2024 Aug 23;16(8):e67566. doi: 10.7759/cureus.67566. eCollection 2024 Aug. Cureus. 2024. PMID: 39310495 Free PMC article.
References
-
- Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the ‘cold shoulder’? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38:399–405. - PubMed
-
- Algwaiz G, Aljurf M, Koh M, et al. Real-World Issues and Potential Solutions in Hematopoietic Cell Transplantation during the COVID-19 Pandemic: Perspectives from the Worldwide Network for Blood and Marrow Transplantation and Center for International Blood and Marrow Transplant Research Health Services and International Studies Committee. Biol Blood Marrow Transplant. 2020;26:2181–2189. - PMC - PubMed
-
- NMD P. Up-to-date information on NMDP/Be The Match response to COVID-19. https://network.bethematchclinical.org/news/nmdp/be-the-match-response-t.... Accessed October 1, 2020.
-
- EBMT. COVID-19 and BMT, EBMT Recommendations. https://www.ebmt.org/sites/default/files/2020-09/EBMT%20COVID-19%20guide.... Accessed October 2, 2020.
-
- Stockschlader M, Hassan HT, Krog C, et al. Long-term follow-up of leukaemia patients after related cryopreserved allogeneic bone marrow transplantation. Br J Haematol. 1997;96:382–386. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

