Decreased Mortality in 1-Year Survivors of Umbilical Cord Blood Transplant vs. Matched Related or Matched Unrelated Donor Transplant in Patients with Hematologic Malignancies
- PMID: 33991725
- PMCID: PMC10234105
- DOI: 10.1016/j.jtct.2021.05.002
Decreased Mortality in 1-Year Survivors of Umbilical Cord Blood Transplant vs. Matched Related or Matched Unrelated Donor Transplant in Patients with Hematologic Malignancies
Abstract
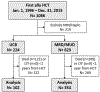
Allogeneic hematopoietic stem cell transplantation (HCT) has the potential to cure hematologic malignancies but is associated with significant morbidity and mortality. Although deaths during the first year after transplantation are often attributable to treatment toxicities and complications, death after the first year may be due to sequelae of accelerated aging caused by cellular senescence. Cytotoxic therapies and radiation used in cancer treatments and conditioning regimens for HCT can induce aging at the molecular level; HCT patients experience time-dependent effects, such as frailty and aging-associated diseases, more rapidly than people who have not been exposed to these treatments. Consistent with this, recipients of younger cells tend to have decreased markers of aging and improved survival, decreased graft-versus-host disease, and lower relapse rates. Given that umbilical cord blood (UCB) is the youngest donor source available, we studied the outcomes after the first year of UCB transplantation versus matched related donor (MRD) and matched unrelated donor (MUD) transplantation in patients with hematologic malignancies over a 20-year period. In this single-center, retrospective study, we examined the outcomes of all adult patients who underwent their first allogeneic HCT through the Duke Adult Bone Marrow Transplant program from January 1, 1996, to December 31, 2015, to allow for at least 3 years of follow-up. Patients were excluded if they died or were lost to follow-up before day 365 after HCT, received an allogeneic HCT for a disease other than a hematologic malignancy, or received cells from a haploidentical or mismatched adult donor. UCB recipients experienced a better unadjusted overall survival than MRD/MUD recipients (log rank P = .03, median overall survival: UCB not reached, MRD/MUD 7.4 years). After adjusting for selected covariates, UCB recipients who survived at least 1 year after HCT had a hazard of death that was 31% lower than that of MRD/MUD recipients (hazard ratio, 0.69; 95% confidence interval, 0.47-0.99; P = .049). This trend held true in a subset analysis of subjects with acute leukemia. UCB recipients also experienced lower rates of moderate or severe chronic graft-versus-host disease (GVHD) and nonrelapse mortality, and slower time to relapse. UCB and MRD/MUD recipients experienced similar rates of grade 2-4 acute GVHD, chronic GHVD, secondary malignancy, and subsequent allogeneic HCT. UCB is already widely used as a donor source in pediatric HCT; however, adult outcomes and adoption have historically lagged behind in comparison. Recent advancements in UCB transplantation such as the implementation of lower-intensity conditioning regimens, double unit transplants, and ex vivo expansion have improved early mortality, making UCB an increasingly attractive donor source for adults; furthermore, our findings suggest that UCB may actually be a preferred donor source for mitigating late effects of HCT.
Keywords: Allogeneic transplant; Mortality; Survivorship; Umbilical cord blood.
Copyright © 2021 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

