How Zinc-Binding Systems, Expressed by Human Pathogens, Acquire Zinc from the Colonized Host Environment: A Critical Review on Zincophores
- PMID: 33992060
- PMCID: PMC9175090
- DOI: 10.2174/1389200222666210514012945
How Zinc-Binding Systems, Expressed by Human Pathogens, Acquire Zinc from the Colonized Host Environment: A Critical Review on Zincophores
Abstract
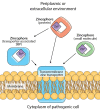
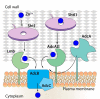
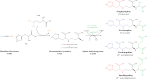
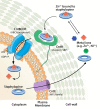
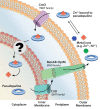
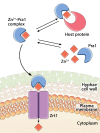
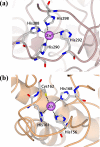
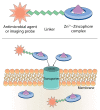
Some transition metals, like manganese, iron, cobalt, nickel, copper and zinc, required for the biosynthesis of metalloenzymes and metalloproteins, are essential micronutrients for the growth and development of pathogenic microorganisms. Among the defenses put in place by the host organism, the so-called "nutritional immunity" consists of reducing the availability of micronutrients and thus "starving" the pathogen. In the case of metals, microorganisms can fight the nutritional immunity in different ways, i.e. by directly recruiting the metal ion or capturing an extracellular metalloprotein or also through the synthesis of specific metallophores which allow importing the metal in the form of a chelate complex. The best known and most studied metallophores are those directed to iron (siderophores), but analogous chelators are also expressed by microorganisms to capture other metals, such as zinc. An efficient zinc recruitment can also be achieved by means of specialized zinc-binding proteins. A deep knowledge of the properties, structure and action mechanisms of extracytoplasmic zinc chelators can be a powerful tool to find out new therapeutic strategies against the antibiotic and/or antifungal resistance. This review aims to collect the knowledge concerning zincophores (small molecules and proteins in charge of zinc acquisition) expressed by bacterial or fungal microorganisms that are pathogenic for the human body.
Keywords: Zincophores; antimicrobial agents.; bacteria; fungi; metal binding proteins; nutritional immunity; zinc.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures


Similar articles
-
Bioinformatic Mapping of Opine-Like Zincophore Biosynthesis in Bacteria.mSystems. 2020 Aug 18;5(4):e00554-20. doi: 10.1128/mSystems.00554-20. mSystems. 2020. PMID: 32817386 Free PMC article.
-
Essential metals at the host-pathogen interface: nutritional immunity and micronutrient assimilation by human fungal pathogens.FEMS Yeast Res. 2015 Nov;15(7):fov071. doi: 10.1093/femsyr/fov071. Epub 2015 Aug 4. FEMS Yeast Res. 2015. PMID: 26242402 Free PMC article. Review.
-
Insights into the Antibacterial Mechanism of Action of Chelating Agents by Selective Deprivation of Iron, Manganese, and Zinc.Appl Environ Microbiol. 2022 Jan 25;88(2):e0164121. doi: 10.1128/AEM.01641-21. Epub 2021 Nov 17. Appl Environ Microbiol. 2022. PMID: 34788072 Free PMC article.
-
Nutritional Immunity and Fungal Pathogenesis: The Struggle for Micronutrients at the Host-Pathogen Interface.Adv Microb Physiol. 2017;70:85-103. doi: 10.1016/bs.ampbs.2017.01.006. Epub 2017 Feb 16. Adv Microb Physiol. 2017. PMID: 28528652 Review.
-
Metalloimmunology: The metal ion-controlled immunity.Adv Immunol. 2020;145:187-241. doi: 10.1016/bs.ai.2019.11.007. Epub 2019 Dec 9. Adv Immunol. 2020. PMID: 32081198 Review.
Cited by
-
Zn2+ and Cu2+ Binding to the Extramembrane Loop of Zrt2, a Zinc Transporter of Candida albicans.Biomolecules. 2022 Jan 12;12(1):121. doi: 10.3390/biom12010121. Biomolecules. 2022. PMID: 35053269 Free PMC article.
-
Pseudogymnoascus destructans Transcriptional Response to Chronic Copper Stress.J Fungi (Basel). 2025 May 13;11(5):372. doi: 10.3390/jof11050372. J Fungi (Basel). 2025. PMID: 40422706 Free PMC article.
-
Is there an association between zinc and COVID-19-associated mucormycosis? Results of an experimental and clinical study.Mycoses. 2021 Oct;64(10):1291-1297. doi: 10.1111/myc.13365. Epub 2021 Sep 1. Mycoses. 2021. PMID: 34420245 Free PMC article.
-
Coordination chemistry suggests that independently observed benefits of metformin and Zn2+ against COVID-19 are not independent.Biometals. 2024 Aug;37(4):983-1022. doi: 10.1007/s10534-024-00590-5. Epub 2024 Apr 5. Biometals. 2024. PMID: 38578560 Free PMC article.
-
The zinc proteome of SARS-CoV-2.Metallomics. 2022 Jul 25;14(7):mfac047. doi: 10.1093/mtomcs/mfac047. Metallomics. 2022. PMID: 35767875 Free PMC article.
References
-
- Rello J., Kalwaje Eshwara V., Conway-Morris A., Lagunes L., Alves J., Alp E., Zhang Z., Mer M., Investigators T.S., TOTEM Study Investigators Perceived differences between intensivists and infectious diseases consultants facing antimicrobial resistance: A global cross-sectional survey. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38(7):1235–1240. doi: 10.1007/s10096-019-03530-1. - DOI - PubMed
-
- European centre for disease prevention and control. Surveillance of antimicrobial resistance in europe. 2018. Available from: www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrob....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

