Immunogenicity and safety of two monovalent rotavirus vaccines, ROTAVAC® and ROTAVAC 5D® in Zambian infants
- PMID: 33992437
- PMCID: PMC8204902
- DOI: 10.1016/j.vaccine.2021.04.060
Immunogenicity and safety of two monovalent rotavirus vaccines, ROTAVAC® and ROTAVAC 5D® in Zambian infants
Abstract
Background and aims: ROTAVAC® (frozen formulation stored at -20 °C) and ROTAVAC 5D® (liquid formulation stable at 2-8 °C) are rotavirus vaccines derived from the 116E human neonatal rotavirus strain, developed and licensed in India. This study evaluated and compared the safety and immunogenicity of these vaccines in an infant population in Zambia.
Methods: We conducted a phase 2b, open-label, randomized, controlled trial wherein 450 infants 6 to 8 weeks of age were randomized equally to receive three doses of ROTAVAC or ROTAVAC 5D, or two doses of ROTARIX®. Study vaccines were administered concomitantly with routine immunizations. Blood samples were collected pre-vaccination and 28 days after the last dose. Serum anti-rotavirus IgA antibodies were measured by ELISA, with WC3 and 89-12 rotavirus strains as viral lysates in the assays. The primary analysis was to assess non-inferiority of ROTAVAC 5D to ROTAVAC in terms of the geometric mean concentration (GMC) of serum IgA (WC3) antibodies. Seroresponse and seropositivity were also determined. Safety was evaluated as occurrence of immediate, solicited, unsolicited, and serious adverse events after each dose.
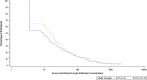
Results: The study evaluated 388 infants in the per-protocol population. All three vaccines were well tolerated and immunogenic. The post-vaccination GMCs were 14.0 U/mL (95% CI: 10.4, 18.8) and 18.1 U/mL (95% CI: 13.7, 24.0) for the ROTAVAC and ROTAVAC 5D groups, respectively, yielding a ratio of 1.3 (95% CI: 0.9, 1.9), thus meeting the pre-set non-inferiority criteria. Solicited and unsolicited adverse events were similar across all study arms. No death or intussusception case was reported during study period.
Conclusions: Among Zambian infants, both ROTAVAC and ROTAVAC 5D were well tolerated and the immunogenicity of ROTAVAC 5D was non-inferior to that of ROTAVAC. These results are consistent with those observed in licensure trials in India and support use of these vaccines across wider geographical areas.
Keywords: Immunogenicity; ROTAVAC; ROTAVAC 5D; Rotavirus Vaccine; Safety; Zambia.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Bhandari N. A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. Journal of Infectious Diseases. 2009;200(3):421–429. - PubMed
-
- Bhandari N. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine. 2014;32(Suppl 1):A110–A116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous