Peer navigation for smoking cessation in smokers with HIV: Protocol for a randomized clinical trial
- PMID: 33992767
- PMCID: PMC8590703
- DOI: 10.1016/j.cct.2021.106435
Peer navigation for smoking cessation in smokers with HIV: Protocol for a randomized clinical trial
Abstract
Background: Smoking prevalence in persons with HIV (PWH) is high (40%) and cessation rates remain low. Lack of social support and poor adherence to nicotine replacement therapy (NRT) are related to poor cessation outcomes; thus, both factors represent possible targets for smoking cessation interventions. Peer navigators (PNs) have been integrated into HIV care with great success to improve engagement and adherence to antiretroviral therapy. However, no clinical trial has evaluated the potential for PNs to provide social support and improve NRT adherence for smoking cessation. We developed a treatment protocol that targets social support, adherence, and self-efficacy for quitting by incorporating PNs into a smoking cessation program. This randomized trial will test whether this approach results in higher rates of 7-day point prevalence abstinence at 12- and 24-weeks, compared to standard treatment.
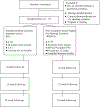
Methods: Seventy-two smokers with HIV will be randomized to either Peer Navigation Social Support for smoking cessation (PNSS-S) or standard cessation counseling. All participants will meet with a nurse for a smoking cessation counseling session, which will include discussion of FDA-approved cessation pharmacotherapy. Participants assigned to PNSS-S will receive weekly phone calls from the PN for 12 weeks. The PN will address readiness to quit, using medication to quit, common barriers to cessation, high risk situations, slip management, and maintaining abstinence. Smoking cessation outcomes will be measured at 4, 12, and 24 weeks following the baseline appointment.
Conclusion: Results from this study will provide preliminary evidence of whether incorporating a peer navigator-based intervention into smoking cessation treatment can improve smoking cessation outcomes in PWH.
Keywords: HIV; Peer navigation; Smoking; Smoking cessation.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of interest declaration
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Proactive text messaging (GetReady2Quit) and nicotine replacement therapy to promote smoking cessation among smokers in primary care: A pilot randomized trial protocol.Contemp Clin Trials. 2019 May;80:48-54. doi: 10.1016/j.cct.2019.03.006. Epub 2019 Mar 25. Contemp Clin Trials. 2019. PMID: 30923022 Free PMC article.
-
The INITIATE trial protocol: a randomized controlled trial testing the effectiveness of a "quit card" intervention on long-term abstinence among tobacco smokers presenting to the emergency department.Trials. 2021 Oct 23;22(1):733. doi: 10.1186/s13063-021-05693-9. Trials. 2021. PMID: 34688291 Free PMC article.
-
Comparative effectiveness of post-discharge strategies for hospitalized smokers: Study protocol for the Helping HAND 4 randomized controlled trial.Trials. 2020 Apr 16;21(1):336. doi: 10.1186/s13063-020-04257-7. Trials. 2020. PMID: 32299470 Free PMC article.
-
Effect of nicotine replacement therapy on quitting by young adults in a trial comparing cessation services.J Public Health Manag Pract. 2014 Mar-Apr;20(2):E7-E15. doi: 10.1097/PHH.0b013e3182a0b8c7. J Public Health Manag Pract. 2014. PMID: 24458316 Free PMC article. Clinical Trial.
-
Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation.Cochrane Database Syst Rev. 2019 Apr 18;4(4):CD013308. doi: 10.1002/14651858.CD013308. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Jun 19;6:CD013308. doi: 10.1002/14651858.CD013308.pub2. PMID: 30997928 Free PMC article. Updated.
Cited by
-
The Impact of the COVID-19 Pandemic on Stress, Isolation, Smoking Behaviors, and Motivation to Quit in People with HIV Who Smoke.AIDS Behav. 2023 Jun;27(6):1862-1869. doi: 10.1007/s10461-022-03917-w. Epub 2022 Nov 11. AIDS Behav. 2023. PMID: 36357808 Free PMC article.
-
Preface to special collection of articles on interventions for promoting smoking cessation among individuals with HIV.Contemp Clin Trials. 2021 Nov;110:106518. doi: 10.1016/j.cct.2021.106518. Epub 2021 Aug 13. Contemp Clin Trials. 2021. PMID: 34400363 Free PMC article. No abstract available.
-
Interventions for tobacco use cessation in people living with HIV.Cochrane Database Syst Rev. 2024 Aug 5;8(8):CD011120. doi: 10.1002/14651858.CD011120.pub3. Cochrane Database Syst Rev. 2024. PMID: 39101506 Free PMC article.
-
Peer Navigation for Smoking Cessation in People With HIV Who Smoke: A Pilot Randomized Controlled Trial.Nicotine Tob Res. 2025 Feb 24;27(3):517-524. doi: 10.1093/ntr/ntae214. Nicotine Tob Res. 2025. PMID: 39251402 Clinical Trial.
References
-
- Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Current opinion in infectious diseases 2013;26:17–25. - PubMed
-
- Ehren K, Hertenstein C, Kummerle T, et al. Causes of death in HIV-infected patients from the Cologne-Bonn cohort. Infection 2014;42:135–40. - PubMed
-
- Current Cigarette Smoking Among Adults in the United States. 2018. (Accessed August 18, 2018, at https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_s....)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical