Understanding insulin and its receptor from their three-dimensional structures
- PMID: 33992784
- PMCID: PMC8513149
- DOI: 10.1016/j.molmet.2021.101255
Understanding insulin and its receptor from their three-dimensional structures
Abstract
Background: Insulin's discovery 100 years ago and its ongoing use since that time to treat diabetes belies the molecular complexity of its structure and that of its receptor. Advances in single-particle cryo-electron microscopy have over the past three years revolutionized our understanding of the atomic detail of insulin-receptor interactions.
Scope of review: This review describes the three-dimensional structure of insulin and its receptor and details on how they interact. This review also highlights the current gaps in our structural understanding of the system.
Major conclusions: A near-complete picture has been obtained of the hormone receptor interactions, providing new insights into the kinetics of the interactions and necessitating a revision of the extant two-site cross-linking model of hormone receptor engagement. How insulin initially engages the receptor and the receptor's traversed trajectory as it undergoes conformational changes associated with activation remain areas for future investigation.
Keywords: Cryo-electron microscopy; Insulin; Insulin receptor; Protein structure; Receptor tyrosine kinase; X-ray crystallography.
Copyright © 2021 The Author. Published by Elsevier GmbH.. All rights reserved.
Figures
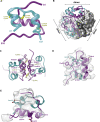



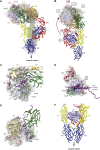
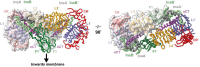
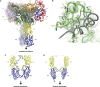


References
-
- Abel J.J., Geiling E.M.K., Rouiller C.A., Bell F.K., Wintersteiner O. Crystalline insulin. Journal of Pharmacology and Experimental Therapeutics. 1927;31(1):65–85.
-
- Crowfoot D. X-ray single crystal photographs of insulin. Nature. 1935;135:591–592.
-
- Crowfoot D., Robinson R. The crystal structure of insulin I. The investigation of air-dried insulin crystals. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 1938;164(919):580–602.
-
- Crowfoot D., Riley D. X-ray measurements on wet insulin crystals. Nature. 1939;144(3659):1011–1012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

