Targeting cholesterol biosynthesis promotes anti-tumor immunity by inhibiting long noncoding RNA SNHG29-mediated YAP activation
- PMID: 33992804
- PMCID: PMC8530930
- DOI: 10.1016/j.ymthe.2021.05.012
Targeting cholesterol biosynthesis promotes anti-tumor immunity by inhibiting long noncoding RNA SNHG29-mediated YAP activation
Abstract
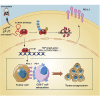
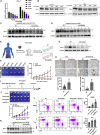
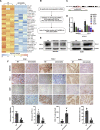
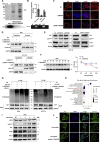
Anti-tumor immunity through checkpoint inhibitors, specifically anti-programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) interaction, is a promising approach for cancer therapy. However, as early clinical trials indicate that colorectal cancers (CRCs) do not respond well to immune-checkpoint therapies, new effective immunotherapy approaches to CRC warrant further study. Simvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (CoA) reductase (HMGCR), the rate-limiting enzyme of the mevalonate (MVA) pathway for the cholesterol biosynthesis. However, little is known about the functions of simvastatin in the regulation of immune checkpoints or long noncoding RNA (lncRNA)-mediated immunoregulation in cancer. Here, we found that simvastatin inhibited PD-L1 expression and promoted anti-tumor immunity via suppressing the expression of lncRNA SNHG29. Interestingly, SNHG29 interacted with YAP and inhibited phosphorylation and ubiquitination-mediated protein degradation of YAP, thereby facilitating downregulation of PD-L1 transcriptionally. Patient-derived tumor xenograft (PDX) models and the clinicopathological analysis in samples from CRC patients further supported the role of the lncRNA SNHG29-mediated PD-L1 signaling axis in tumor microenvironment reprogramming. Collectively, our study uncovers simvastatin as a potential therapeutic drug for immunotherapy in CRC, which suppresses lncRNA SNHG29-mediated YAP activation and promotes anti-tumor immunity by inhibiting PD-L1 expression.
Keywords: YAP activation; colorectal cancer; immune-checkpoint PD-L1; immunotherapy; lncRNA SNHG29; simvastatin.
Copyright © 2021 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer.J Immunother Cancer. 2021 Jan;9(1):e001895. doi: 10.1136/jitc-2020-001895. J Immunother Cancer. 2021. PMID: 33462141 Free PMC article.
-
LncRNA HCG18 suppresses CD8+ T cells to confer resistance to cetuximab in colorectal cancer via miR-20b-5p/PD-L1 axis.Epigenomics. 2021 Aug;13(16):1281-1297. doi: 10.2217/epi-2021-0130. Epub 2021 Sep 15. Epigenomics. 2021. PMID: 34523356
-
Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3.Mol Cancer. 2019 Oct 16;18(1):143. doi: 10.1186/s12943-019-1079-y. Mol Cancer. 2019. PMID: 31619268 Free PMC article.
-
The potential role of PD-1/PD-L1 small molecule inhibitors in colorectal cancer with different mechanisms of action.Eur J Pharmacol. 2025 Apr 5;992:177351. doi: 10.1016/j.ejphar.2025.177351. Epub 2025 Feb 6. Eur J Pharmacol. 2025. PMID: 39922421 Review.
-
PD-1/PD-L1-dependent immune response in colorectal cancer.J Cell Physiol. 2020 Jul;235(7-8):5461-5475. doi: 10.1002/jcp.29494. Epub 2020 Jan 21. J Cell Physiol. 2020. PMID: 31960962 Review.
Cited by
-
ATR/Chk1 interacting lncRNA modulates DNA damage response to induce breast cancer chemoresistance.Cell Insight. 2024 Jul 14;3(5):100183. doi: 10.1016/j.cellin.2024.100183. eCollection 2024 Oct. Cell Insight. 2024. PMID: 39148723 Free PMC article.
-
Clinical potential of the Hippo-YAP pathway in bladder cancer.Front Oncol. 2022 Jul 15;12:925278. doi: 10.3389/fonc.2022.925278. eCollection 2022. Front Oncol. 2022. PMID: 35912245 Free PMC article. Review.
-
Patient-derived xenograft model in cancer: establishment and applications.MedComm (2020). 2025 Jan 19;6(2):e70059. doi: 10.1002/mco2.70059. eCollection 2025 Feb. MedComm (2020). 2025. PMID: 39830019 Free PMC article. Review.
-
PCSK9 Manipulates Lipid Metabolism and the Immune Microenvironment in Cancer.Onco Targets Ther. 2025 Mar 27;18:411-427. doi: 10.2147/OTT.S504637. eCollection 2025. Onco Targets Ther. 2025. PMID: 40166624 Free PMC article. Review.
-
The roles and molecular mechanisms of non-coding RNA in cancer metabolic reprogramming.Cancer Cell Int. 2024 Jan 18;24(1):37. doi: 10.1186/s12935-023-03186-0. Cancer Cell Int. 2024. PMID: 38238756 Free PMC article. Review.
References
-
- Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394:1467–1480. - PubMed
-
- Punt C.J., Koopman M., Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2017;14:235–246. - PubMed
-
- Farooqi A.A., de la Roche M., Djamgoz M.B.A., Siddik Z.H. Overview of the oncogenic signaling pathways in colorectal cancer: Mechanistic insights. Semin. Cancer Biol. 2019;58:65–79. - PubMed
-
- Crockett S.D., Nagtegaal I.D. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. 2019;157:949–966.e4. - PubMed
-
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

