Breaking androgen receptor addiction of prostate cancer by targeting different functional domains in the treatment of advanced disease
- PMID: 33993099
- PMCID: PMC8138777
- DOI: 10.1016/j.tranon.2021.101115
Breaking androgen receptor addiction of prostate cancer by targeting different functional domains in the treatment of advanced disease
Abstract
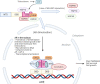
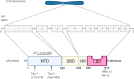
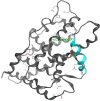
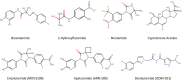
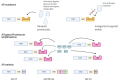
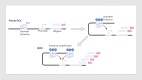
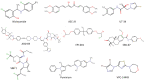
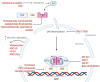
In the last decade, treatment for castration-resistant prostate cancer has changed markedly, impacting symptom control and longevity for patients. However, a large proportion of cases progress despite androgen deprivation therapy and chemotherapy, while still being fit enough for several more lines of treatment. Overstimulation of the androgen receptor (AR) activity is the main driver of this cancer. Targeting biological functions of the AR or its co-regulators has proven very effective in this disease and led to the development of several highly effective drugs targeting the AR signalling axis. Drugs such as enzalutamide demonstrated that the improvement in anti-tumour efficacy is closely correlated with an affinity for the AR and its activity and have established the paradigm that AR remains activity in aggressive disease. However, as importantly, key insights into mechanisms of resistance are guiding the development of the next generation of AR-targeted drugs. This review outlines the historical development of these highly specific agents, their mechanism of action in the context of defective AR activity, and explores the potential for the upcoming next-generation AR inhibitors (ARI) for prostate cancer by targeting the alternative domains of AR, rather than by the conventional ligand-binding domain approach. There is huge potential in these approaches to develop new drugs with high clinical activity and further improve the outlook for patients.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures


References
-
- Cancer Research UK, "Prostate cancer statistics," 2017. [Online]. Available: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s.... [Accessed 30 June 2020].
-
- Ingham M., Lee R., MacDermed D., Olumi A. Trans women and prostate cancer. Urology. July 2020;36(12):518–525. - PubMed
-
- World Health Organization: International Agency for Research on Cancer, "Global cancer observatory: cancer today," 2018. [Online]. Available: https://gco.iarc.fr/. [Accessed 6 November 2020].
-
- Prostate Cancer UK, "Best practice pathway," June 2019. [Online]. Available: https://prostatecanceruk.org/about-us/projects-and-policies/best-practic.... [Accessed 4 11 2020].
-
- American Cancer Society, "Survival rates for prostate cancer," 9 January 2020. [Online]. Available: https://www.cancer.org/content/dam/CRC/PDF/Public/8795.00.pdf. [Accessed 6 November 2020].
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

