HIV drug resistance among adults initiating antiretroviral therapy in Uganda
- PMID: 33993252
- PMCID: PMC8361362
- DOI: 10.1093/jac/dkab159
HIV drug resistance among adults initiating antiretroviral therapy in Uganda
Abstract
Background: WHO revised their HIV drug resistance (HIVDR) monitoring strategy in 2014, enabling countries to generate nationally representative HIVDR prevalence estimates from surveys conducted using this methodology. In 2016, we adopted this strategy in Uganda and conducted an HIVDR survey among adults initiating or reinitiating ART.
Methods: A cross-sectional survey of adults aged ≥18 years initiating or reinitiating ART was conducted at 23 sites using a two-stage cluster design sampling method. Participants provided written informed consent prior to enrolment. Whole blood collected in EDTA vacutainer tubes was used for preparation of dried blood spot (DBS) specimens or plasma. Samples were shipped from the sites to the Central Public Health Laboratory (CPHL) for temporary storage before transfer to the Uganda Virus Research Institute (UVRI) for genotyping. Prevalence of HIVDR among adults initiating or reinitiating ART was determined.
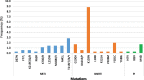
Results: Specimens from 491 participants (median age 32 years and 61.5% female) were collected between August and December 2016. Specimens from 351 participants were successfully genotyped. Forty-nine had drug resistance mutations, yielding an overall weighted HIVDR prevalence of 18.2% with the highest noted for NNRTIs at 14.1%.
Conclusions: We observed a high HIVDR prevalence for NNRTIs among adults prior to initiating or reinitiating ART in Uganda. This is above WHO's recommended threshold of 10% when countries should consider changing from NNRTI- to dolutegravir-based first-line regimens. This recommendation was adopted in the revised Ugandan ART guidelines. Dolutegravir-containing ART regimens are preferred for first- and second-line ART regimens.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures
References
-
- WHO. WHO guidelines on the Public Health Response to Pretreatment HIV Drug Resistance. 2017. https://www.who.int/hiv/pub/guidelines/hivdr-guidelines-2017/en/.
-
- Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 1995; 267: 483–9. - PubMed
-
- Cambiano V, Bertagnolio S, Jordan MR et al. Predicted levels of HIV drug resistance: potential impact of expanding diagnosis, retention, and eligibility criteria for antiretroviral therapy initiation. AIDS 2014; 28 Suppl 1: S15–23. - PubMed
-
- WHO. WHO HIV Drug Resistance Report. 2012. http://apps.who.int/iris/bitstream/10665/75183/1/9789241503938_eng.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

