Selective involution of thymic medulla by cyclosporine A with a decrease of mature thymic epithelia, XCR1+ dendritic cells, and epithelium-free areas containing Foxp3+ thymic regulatory T cells
- PMID: 33993340
- PMCID: PMC8397693
- DOI: 10.1007/s00418-021-01993-y
Selective involution of thymic medulla by cyclosporine A with a decrease of mature thymic epithelia, XCR1+ dendritic cells, and epithelium-free areas containing Foxp3+ thymic regulatory T cells
Abstract
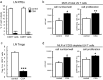
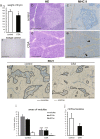
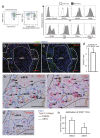
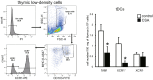
Immunosuppressive drugs such as cyclosporine A (CSA) can disrupt thymic structure and functions, ultimately inducing syngeneic/autologous graft-versus-host disease together with involuted medullas. To elucidate the effects of CSA on the thymus more precisely, we analyzed the effects of CSA on the thymus and T cell system using rats. In addition to confirming the phenomena already reported, we newly found that the proportion of recent thymic emigrants also greatly decreased, suggesting impaired supply. Immunohistologically, the medullary thymic epithelial cells (mTECs) presented with a relative decrease in the subset with a competent phenotype and downregulation of class II major histocompatibility complex molecules. In control rats, thymic dendritic cells (DCs) comprised two subsets, XCR1+SIRP1α-CD4- and XCR1-SIRP1α+CD4+. The former had a tendency to selectively localize in the previously-reported epithelium-containing areas of the rat medullas, and the number was significantly reduced by CSA treatment. The epithelium-free areas, another unique domains in the rat medullas, contained significantly more Foxp3+ thymic Tregs. With CSA treatment, the epithelium-free areas presented strong involution, and the number and distribution of Tregs in the medulla were greatly reduced. These results suggest that CSA inhibits the production of single-positive thymocytes, including Tregs, and disturbs the microenvironment of the thymic medulla, with a decrease of the competent mTECs and disorganization of epithelium-free areas and DC subsets, leading to a generation of autoreactive T cells with selective medullary involution.
Keywords: Dendritic cells; Immunosuppressive drug; Regulatory T cells; Thymic epithelial cells; Thymic structure; Thymus.
© 2021. The Author(s).
Conflict of interest statement
All authors declare that no support, financial or otherwise, has been received from any organization that may have an interest in the submitted work, and there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


Similar articles
-
Differential effects of X-irradiation and cyclosporin-A administration on the thymus with respect to the generation of cyclosporin-A-induced autoimmunity.Dev Immunol. 1995;4(2):127-38. doi: 10.1155/1995/18495. Dev Immunol. 1995. PMID: 9700362 Free PMC article.
-
Recruitment of semiallogeneic dendritic cells to the thymus during post-cyclosporine thymic regeneration.Transplantation. 1995 Dec 15;60(11):1326-30. Transplantation. 1995. PMID: 8525529
-
Enhancement of thymic recovery after cyclosporine by recombinant human growth hormone and insulin-like growth factor I.Transplantation. 1991 Nov;52(5):879-84. doi: 10.1097/00007890-199111000-00024. Transplantation. 1991. PMID: 1949174
-
Cyclosporin A-induced changes of the thymic microenvironment. A review of morphological studies.Histol Histopathol. 1998 Oct;13(4):1183-96. doi: 10.14670/HH-13.1183. Histol Histopathol. 1998. PMID: 9810510 Review.
-
Differential effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin, bis(tri-n-butyltin) oxide and cyclosporine on thymus histophysiology.Crit Rev Toxicol. 1997 Jul;27(4):381-430. doi: 10.3109/10408449709089900. Crit Rev Toxicol. 1997. PMID: 9263645 Review.
Cited by
-
Current Status of Adverse Event Profile of Cyclosporine in Kidney, Stem Cell, and Heart Transplantations Using the Japanese Pharmacovigilance Database.Cureus. 2022 Sep 20;14(9):e29383. doi: 10.7759/cureus.29383. eCollection 2022 Sep. Cureus. 2022. PMID: 36159360 Free PMC article.
-
A Proposed Link Between Acute Thymic Involution and Late Adverse Effects of Chemotherapy.Front Immunol. 2022 Jul 1;13:933547. doi: 10.3389/fimmu.2022.933547. eCollection 2022. Front Immunol. 2022. PMID: 35844592 Free PMC article.
References
-
- Anderson G, Anderson KL, Conroy LA, Hallam TJ, Moore NC, Owen JJT, Jenkinson EJ. Intracellular signaling events during positive and negative selection of CD4+CD8+ thymocytes in vitro. J Immunol. 1995;154(8):3636–3643. - PubMed
-
- Chen WR, Thoburn C, Hess AD. Characterization of the pathogenic autoreactive T cells in cyclosporine-induced syngeneic graft-versus-host disease. J Immunol. 1998;161(12):7040–7046. - PubMed
-
- Cheney RT, Sprent J. Capacity of cyclosporine to induce auto-graft-versus-host disease and impair intrathymic T cell differentiation. Transplant Proc. 1985;17(1):528–530.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

