Genetic testing in patients undergoing percutaneous coronary intervention: rationale, evidence and practical recommendations
- PMID: 33993817
- PMCID: PMC9008593
- DOI: 10.1080/17512433.2021.1927709
Genetic testing in patients undergoing percutaneous coronary intervention: rationale, evidence and practical recommendations
Abstract
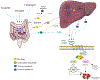
Introduction: Clopidogrel is the most frequently utilized P2Y12 inhibitor and is characterized by broad interindividual response variability resulting in impaired platelet inhibition and increased risk of thrombotic complications in a considerable number of patients. The potent P2Y12 inhibitors, prasugrel and ticagrelor, can overcome this limitation but at the expense of an increased risk of bleeding. Genetic variations of the cytochrome P450 (CYP) 2 C19 enzyme, a key determinant in clopidogrel metabolism, have been strongly associated with clopidogrel response profiles prompting investigations of genetic-guided selection of antiplatelet therapy.
Areas covered: The present manuscript focuses on the rationale for the use of genetic testing to guide the selection of platelet P2Y12 inhibitors among patients undergoing percutaneous coronary intervention (PCI). Moreover, a comprehensive appraisal of the available evidence and practical recommendations is provided.
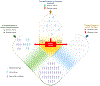
Expert commentary: Implementation of genetic testing as a strategy to guide the selection of therapy can result in escalation (i.e. switching to prasugrel or ticagrelor) or de-escalation (i.e. switching to clopidogrel) of P2Y12 inhibiting therapy. Most recent investigations support the clinical benefit of a genetic guided selection of antiplatelet therapy in patients undergo PCI. Integrating the results of genetic testing with clinical and procedural variables represents a promising strategy for a precision medicine approach for the selection of antiplatelet therapy among patients undergoing PCI.
Keywords: Genetics; antiplatelet therapy; bleeding; p2y12 inhibitors; percutaneous coronary intervention; thrombosis.
Figures


References
-
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018. Jan 7;39(2):119–177. doi: 10.1093/eurheartj/ehx393. - DOI - PubMed
-
- Bossavy JP, Thalamas C, Sagnard L, et al. A double-blind randomized comparison of combined aspirin and ticlopidine therapy versus aspirin or ticlopidine alone on experimental arterial thrombogenesis in humans. Blood. 1998. Sep 1;92(5):1518–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
