High-fat diet impairs duodenal barrier function and elicits glia-dependent changes along the gut-brain axis that are required for anxiogenic and depressive-like behaviors
- PMID: 33993886
- PMCID: PMC8126158
- DOI: 10.1186/s12974-021-02164-5
High-fat diet impairs duodenal barrier function and elicits glia-dependent changes along the gut-brain axis that are required for anxiogenic and depressive-like behaviors
Abstract
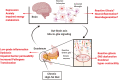
Background: Mood and metabolic disorders are interrelated and may share common pathological processes. Autonomic neurons link the brain with the gastrointestinal tract and constitute a likely pathway for peripheral metabolic challenges to affect behaviors controlled by the brain. The activities of neurons along these pathways are regulated by glia, which exhibit phenotypic shifts in response to changes in their microenvironment. How glial changes might contribute to the behavioral effects of consuming a high-fat diet (HFD) is uncertain. Here, we tested the hypothesis that anxiogenic and depressive-like behaviors driven by consuming a HFD involve compromised duodenal barrier integrity and subsequent phenotypic changes to glia and neurons along the gut-brain axis.
Methods: C57Bl/6 male mice were exposed to a standard diet or HFD for 20 weeks. Bodyweight was monitored weekly and correlated with mucosa histological damage and duodenal expression of tight junction proteins ZO-1 and occludin at 0, 6, and 20 weeks. The expression of GFAP, TLR-4, BDNF, and DCX were investigated in duodenal myenteric plexus, nodose ganglia, and dentate gyrus of the hippocampus at the same time points. Dendritic spine number was measured in cultured neurons isolated from duodenal myenteric plexuses and hippocampi at weeks 0, 6, and 20. Depressive and anxiety behaviors were also assessed by tail suspension, forced swimming, and open field tests.
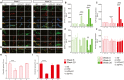
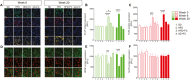
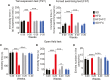
Results: HFD mice exhibited duodenal mucosa damage with marked infiltration of immune cells and decreased expression of ZO-1 and occludin that coincided with increasing body weight. Glial expression of GFAP and TLR4 increased in parallel in the duodenal myenteric plexuses, nodose ganglia, and hippocampus in a time-dependent manner. Glial changes were associated with a progressive decrease in BDNF, and DCX expression, fewer neuronal dendritic spines, and anxiogenic/depressive symptoms in HFD-treated mice. Fluorocitrate (FC), a glial metabolic poison, abolished these effects both in the enteric and central nervous systems and prevented behavioral alterations at week 20.
Conclusions: HFD impairs duodenal barrier integrity and produces behavioral changes consistent with depressive and anxiety phenotypes. HFD-driven changes in both peripheral and central nervous systems are glial-dependent, suggesting a potential glial role in the alteration of the gut-brain signaling that occurs during metabolic disorders and psychiatric co-morbidity.
Keywords: Behavioral disorders; Enteric glia; Glial signaling; Gut-brain axis; High-fat diet; Intestinal hyper-permeability.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Oleoylethanolamide-producing Lactobacillus paracasei F19 improves metabolic and behavioral disorders by restoring intestinal permeability and microbiota-gut-brain axis in high-fat diet-induced obese male mice.Brain Behav Immun. 2025 Jul;127:25-44. doi: 10.1016/j.bbi.2025.02.014. Epub 2025 Feb 21. Brain Behav Immun. 2025. PMID: 39988008
-
High-fat diet-induced alterations to gut microbiota and gut-derived lipoteichoic acid contributes to the development of enteric neuropathy.Neurogastroenterol Motil. 2020 Jul;32(7):e13838. doi: 10.1111/nmo.13838. Epub 2020 Mar 13. Neurogastroenterol Motil. 2020. PMID: 32168415 Free PMC article.
-
High-Fat Diet During the Perinatal Period Induces Loss of Myenteric Nitrergic Neurons and Increases Enteric Glial Density, Prior to the Development of Obesity.Neuroscience. 2018 Nov 21;393:369-380. doi: 10.1016/j.neuroscience.2018.09.033. Neuroscience. 2018. PMID: 30454864 Free PMC article.
-
Chronic stress-induced gut dysfunction exacerbates Parkinson's disease phenotype and pathology in a rotenone-induced mouse model of Parkinson's disease.Neurobiol Dis. 2020 Feb;135:104352. doi: 10.1016/j.nbd.2018.12.012. Epub 2018 Dec 21. Neurobiol Dis. 2020. PMID: 30579705 Review.
-
The Physiology of Enteric Glia.Annu Rev Physiol. 2025 Feb;87(1):353-380. doi: 10.1146/annurev-physiol-022724-105016. Epub 2025 Feb 3. Annu Rev Physiol. 2025. PMID: 39546562 Review.
Cited by
-
Pterostilbene mediates glial and immune responses to alleviate chronic intermittent hypoxia-induced oxidative stress in nerve cells.PLoS One. 2023 Jun 2;18(6):e0286686. doi: 10.1371/journal.pone.0286686. eCollection 2023. PLoS One. 2023. PMID: 37267263 Free PMC article.
-
Enteric glial NLRP3 inflammasome contributes to gut mucosal barrier alterations in a mouse model of diet-induced obesity.Acta Physiol (Oxf). 2025 Jan;241(1):e14232. doi: 10.1111/apha.14232. Epub 2024 Sep 17. Acta Physiol (Oxf). 2025. PMID: 39287080 Free PMC article.
-
Functional Intraregional and Interregional Heterogeneity between Myenteric Glial Cells of the Colon and Duodenum in Mice.J Neurosci. 2022 Nov 16;42(46):8694-8708. doi: 10.1523/JNEUROSCI.2379-20.2022. Epub 2022 Nov 1. J Neurosci. 2022. PMID: 36319118 Free PMC article.
-
The enteric nervous system.Physiol Rev. 2023 Apr 1;103(2):1487-1564. doi: 10.1152/physrev.00018.2022. Epub 2022 Dec 15. Physiol Rev. 2023. PMID: 36521049 Free PMC article. Review.
-
Effects of the Lipid Metabolites and the Gut Microbiota in ApoE-/- Mice on Atherosclerosis Co-Depression From the Microbiota-Gut-Brain Axis.Front Mol Biosci. 2022 Apr 26;9:786492. doi: 10.3389/fmolb.2022.786492. eCollection 2022. Front Mol Biosci. 2022. PMID: 35558553 Free PMC article.
References
-
- Fournel A, Drougard A, Duparc T, Marlin A, Brierley SM, Castro J, le-Gonidec S, Masri B, Colom A, Lucas A, Rousset P, Cenac N, Vergnolle N, Valet P, Cani PD, Knauf C. Apelin targets gut contraction to control glucose metabolism via the brain. Gut. 2017;66(2):258–269. doi: 10.1136/gutjnl-2015-310230. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

