Fetal Goitrous Hyperthyroidism in a Pregnant Woman with Triiodothyronine-Predominant Graves' Disease
- PMID: 33994519
- PMCID: PMC8212807
Fetal Goitrous Hyperthyroidism in a Pregnant Woman with Triiodothyronine-Predominant Graves' Disease
Abstract
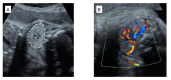
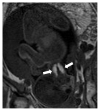
Triiodothyronine (T3)-predominant Graves' disease is characterized by increased serum free T3 (FT3) levels after free thyroxine (FT4) levels become normal or even low during antithyroid drug treatment. We encountered a 34-year-old pregnant woman, gravida 5 para 4, who was complicated by T3-predominant Graves' disease. She was diagnosed with Graves' disease at 20 years old, and had received methimazole. Methimazole was changed to potassium iodide to reduce the risk of congenital anomalies during the first trimester. The dose of antithyroid drugs was adjusted based on maternal FT4 levels, so that maternal Graves' disease deteriorated and fetal goitrous hyperthyroidism appeared during the second trimester. Since the fetus presented goiter and tachycardia at 27-28 gestational weeks, doses of methimazole and potassium iodide were increased. A male newborn weighing 2604 g was delivered by a cesarean section at 35 gestational weeks. The newborn was diagnosed with neonatal hyperthyroidism, and received methimazole for six months. He developed normally with normal thyroid function at 1 year old. In pregnancies complicated by T3-predominant Graves' disease, the kinds and doses of antithyroid drugs have to be carefully selected to maintain maternal levels of FT4 as well as FT3 within the normal range, considering trimesters of pregnancy, teratogenicity of medication, and maternal levels of thyroid-stimulating hormone receptor antibody.
Keywords: Graves' disease; antithyroid drug; fetal goiter; fetal hyperthyroidism; tachycardia.
Figures



Similar articles
-
Fetal goiter identified in a pregnant woman with triiodothyronine-predominant graves' disease: a case report.BMC Pregnancy Childbirth. 2020 Jun 3;20(1):344. doi: 10.1186/s12884-020-03035-2. BMC Pregnancy Childbirth. 2020. PMID: 32493403 Free PMC article.
-
Graves' disease in two pregnancies complicated by fetal goitrous hypothyroidism: successful in utero treatment with levothyroxine.Thyroid. 2011 Jan;21(1):75-81. doi: 10.1089/thy.2010.0286. Epub 2010 Dec 16. Thyroid. 2011. PMID: 21162688
-
Substituting Potassium Iodide for Methimazole as the Treatment for Graves' Disease During the First Trimester May Reduce the Incidence of Congenital Anomalies: A Retrospective Study at a Single Medical Institution in Japan.Thyroid. 2015 Oct;25(10):1155-61. doi: 10.1089/thy.2014.0581. Epub 2015 Aug 28. Thyroid. 2015. PMID: 26222916
-
[Treatment of hyperthyroidism due to Graves' disease: what is the recommended antithyroid drug during pregnancy?].J Gynecol Obstet Biol Reprod (Paris). 2013 May;42(3):232-7. doi: 10.1016/j.jgyn.2012.11.012. Epub 2013 Jan 9. J Gynecol Obstet Biol Reprod (Paris). 2013. PMID: 23312275 Review. French.
-
[Current problems in the treatment of Graves' disease in pregnancy and in lactation].Nihon Rinsho. 2006 Dec;64(12):2297-302. Nihon Rinsho. 2006. PMID: 17154095 Review. Japanese.
References
-
- Andersen SL, Olsen J, Wu CS, Laurberg P. Birth defects after early pregnancy use of antithyroid drugs: a Danish nationawide study. J Clin Endocrinol Metab. 2013;98:4373–81. - PubMed
-
- Cooper DS, Rivkees SA. Putting propylthiouracil perspective. J Clin Endocrinol Metab. 2009;94:1881–2. - PubMed
-
- Corral E, Reascos M, Preiss Y, Rompel SM, Sepulveda W. Treatment of fetal goitrous hypothyroidism: value of direct intramuscular L-thyroxine therapy. Prenat Diagn. 2010;30:899–901. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

