Protein kinase CK2: a potential therapeutic target for diverse human diseases
- PMID: 33994545
- PMCID: PMC8126563
- DOI: 10.1038/s41392-021-00567-7
Protein kinase CK2: a potential therapeutic target for diverse human diseases
Abstract
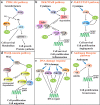
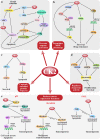
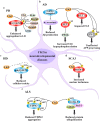
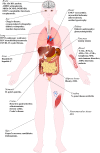
CK2 is a constitutively active Ser/Thr protein kinase, which phosphorylates hundreds of substrates, controls several signaling pathways, and is implicated in a plethora of human diseases. Its best documented role is in cancer, where it regulates practically all malignant hallmarks. Other well-known functions of CK2 are in human infections; in particular, several viruses exploit host cell CK2 for their life cycle. Very recently, also SARS-CoV-2, the virus responsible for the COVID-19 pandemic, has been found to enhance CK2 activity and to induce the phosphorylation of several CK2 substrates (either viral and host proteins). CK2 is also considered an emerging target for neurological diseases, inflammation and autoimmune disorders, diverse ophthalmic pathologies, diabetes, and obesity. In addition, CK2 activity has been associated with cardiovascular diseases, as cardiac ischemia-reperfusion injury, atherosclerosis, and cardiac hypertrophy. The hypothesis of considering CK2 inhibition for cystic fibrosis therapies has been also entertained for many years. Moreover, psychiatric disorders and syndromes due to CK2 mutations have been recently identified. On these bases, CK2 is emerging as an increasingly attractive target in various fields of human medicine, with the advantage that several very specific and effective inhibitors are already available. Here, we review the literature on CK2 implication in different human pathologies and evaluate its potential as a pharmacological target in the light of the most recent findings.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- IG 18756/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 22974/AFM-Téléthon (French Muscular Dystrophy Association)
- FFC#12/2017/Fondazione per la Ricerca sulla Fibrosi Cistica (Fondazione FFC)
- FFC#11/2019/Fondazione per la Ricerca sulla Fibrosi Cistica (Fondazione FFC)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

