Between life and death: strategies to reduce phototoxicity in super-resolution microscopy
- PMID: 33994582
- PMCID: PMC8114953
- DOI: 10.1088/1361-6463/ab6b95
Between life and death: strategies to reduce phototoxicity in super-resolution microscopy
Abstract
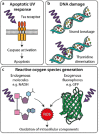
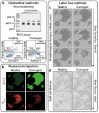
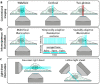
Super-resolution microscopy (SRM) enables non-invasive, molecule-specific imaging of the internal structure and dynamics of cells with sub-diffraction limit spatial resolution. One of its major limitations is the requirement for high-intensity illumination, generating considerable cellular phototoxicity. This factor considerably limits the capacity for live-cell observations, particularly for extended periods of time. Here, we give an overview of new developments in hardware, software and probe chemistry aiming to reduce phototoxicity. Additionally, we discuss how the choice of biological model and sample environment impacts the capacity for live-cell observations.
Keywords: fluorescence; photodamage; phototoxicity; super-resolution microscopy.
© 2020 IOP Publishing Ltd.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
