Total synthesis of (-)-4-desacetoxy-1-oxovindoline: Single atom exchange of an embedded core heteroatom in vindoline
- PMID: 33994597
- PMCID: PMC8117404
- DOI: 10.1016/j.tet.2021.132117
Total synthesis of (-)-4-desacetoxy-1-oxovindoline: Single atom exchange of an embedded core heteroatom in vindoline
Abstract
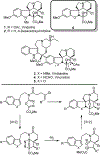
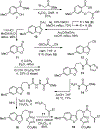
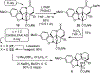
A concise total synthesis of (-)-4-desacetoxy-1-oxovindoline is disclosed, bearing a single heteroatom exchange in the core structure of the natural product 4-desacetoxyvindoline. Central to the synthesis is powerful oxadiazole intramolecular [4+2]/[3+2] cycloaddition cascade that formed four C-C bonds, created three new rings, and established five contiguous stereocenters about the new formed central 6-membered ring.
Keywords: Vinca alkaloids; oxadiazole cycloaddition cascade; vindoline analogue.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reported in this paper.
Figures



Similar articles
-
Total synthesis of (-)- and ent-(+)-vindoline and related alkaloids.J Am Chem Soc. 2006 Aug 16;128(32):10596-612. doi: 10.1021/ja061256t. J Am Chem Soc. 2006. PMID: 16895428 Free PMC article.
-
Total synthesis of natural (+)- [corrected] and ent-(-)-4-desacetoxy-6,7-dihydrovindorosine [corrected] and natural and ent-minovine: oxadiazole tandem intramolecular Diels-Alder/1,3-dipolar cycloaddition reaction.Org Lett. 2005 Feb 17;7(4):741-4. doi: 10.1021/ol050017s. Org Lett. 2005. PMID: 15704939 Free PMC article.
-
Asymmetric total synthesis of vindoline.J Am Chem Soc. 2010 Mar 24;132(11):3685-7. doi: 10.1021/ja910695e. J Am Chem Soc. 2010. PMID: 20187641 Free PMC article.
-
Asymmetric total synthesis of vindorosine, vindoline, and key vinblastine analogues.J Am Chem Soc. 2010 Sep 29;132(38):13533-44. doi: 10.1021/ja106284s. J Am Chem Soc. 2010. PMID: 20809620 Free PMC article.
-
Total synthesis of (-)- and ent-(+)-vindoline.Org Lett. 2005 Sep 29;7(20):4539-42. doi: 10.1021/ol051975x. Org Lett. 2005. PMID: 16178578 Free PMC article.
References
-
- The Alkaloids; Brossi A, Suffness M, Eds.; Academic: San Diego, 1990, Vol. 37.
-
- Neuss N, Barnes AJ Jr., Huckstep LL, Experientia 31 (1975) 18. - PubMed
-
- Barnett CJ, Cullinan GJ, Gerzon K, Hoying BC, Jones WE, Newlon WM, Poore GA, Robison RL, Sweeney MJ, Todd GC, Dyke RW, Nelson RL, Med RLJ. Chem 21 (1978) 88. - PubMed
-
- Lobert S, Vulevic B, Correia JJ, Biochemistry 35 (1996) 6806. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

