Small Extracellular Vesicles Derived from Adipose Tissue Prevent Bisphosphonate-Related Osteonecrosis of the Jaw by Promoting Angiogenesis
- PMID: 33994785
- PMCID: PMC8114828
- DOI: 10.2147/IJN.S305361
Small Extracellular Vesicles Derived from Adipose Tissue Prevent Bisphosphonate-Related Osteonecrosis of the Jaw by Promoting Angiogenesis
Abstract
Purpose: There is no definitive treatment for bisphosphonate-related osteonecrosis of the jaw (BRONJ). Small extracellular vesicles derived from adipose tissue (sEV-AT) have been proved efficient at promoting tissue regeneration. The aim of this study was to evaluate the effects of sEV-AT administration on BRONJ-like lesions in rats.
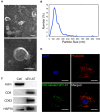
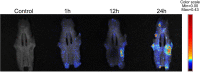
Methods: Zoledronate (Zol) and dexamethasone (Dex) were subcutaneously administered to create a BRONJ rat model. Rats were randomly divided into three groups: 1) Control; 2) Zol+Dex; 3) sEV-AT. The maxillary left first molars were extracted two weeks after the first administration. In the sEV-AT group, sEV-AT were given intravenously every three days right after tooth extraction. We preformed occlusal view images, microcomputed tomography (µCT) and histological analysis to measure the regeneration of osseous and soft tissue in extraction sockets. Human umbilical vein endothelial cells (HUVECs) were isolated and cultured with endothelial cell medium (ECM). HUVECs were then divided into three groups: 1) Control: ECM; 2) Zol: ECM+Zol; 3) sEV-AT: ECM+Zol+sEV-AT. We evaluated the proliferation, tube formation and migration of HUVECs in each group.
Results: Rats treated with Zol+Dex showed BRONJ-like lesions including open wounds, necrotic bones, empty osteocyte lacunae and reduced osteoclasts. sEV-AT administration reduced BRONJ-like lesions by promoting soft tissue healing. µCT results showed that bone volume in extraction sockets in the sEV-AT group was larger than the Zol+Dex group. Histological analysis showed less necrotic bones and empty osteocyte lacunae in the sEV-AT group compared to the Zol+Dex group. Histological analysis also showed more osteoclasts, collagen fibers and blood vessels in the sEV-AT group compared to the Zol+Dex group. Furthermore, sEV-AT enhanced the proliferation, migration and tube formation of HUVECs which were inhibited by Zol.
Conclusion: Our findings indicate that sEV-AT prevent BRONJ in rats. Angiogenesis promotion contributes to the prevention of BRONJ.
Keywords: adipose tissue; angiogenesis; bisphosphonate-related osteonecrosis of the jaw; endothelial cells; small extracellular vesicles.
© 2021 Huang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- Ramaglia L, Guida A, Iorio-Siciliano V, Cuozzo A, Blasi A, Sculean A. Stage-specific therapeutic strategies of medication-related osteonecrosis of the jaws: a systematic review and meta-analysis of the drug suspension protocol. Clin Oral Investig. 2018;22(2):597–615. doi: 10.1007/s00784-017-2325-6 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

