Accelerated Bone Regeneration by Astragaloside IV through Stimulating the Coupling of Osteogenesis and Angiogenesis
- PMID: 33994865
- PMCID: PMC8120474
- DOI: 10.7150/ijbs.57681
Accelerated Bone Regeneration by Astragaloside IV through Stimulating the Coupling of Osteogenesis and Angiogenesis
Abstract
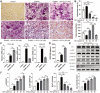
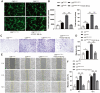
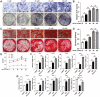
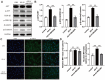
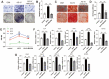
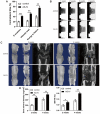
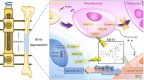
Both osteoblasts and preosteoclasts contribute to the coupling of osteogenesis and angiogenesis, regulating bone regeneration. Astragaloside IV (AS-IV), a glycoside of cycloartane-type triterpene derived from the Chinese herb Astragalus membranaceus, exhibits various biological activities, including stimulating angiogenesis and attenuating ischemic-hypoxic injury. However, the effects and underlying mechanisms of AS-IV in osteogenesis, osteoclastogenesis, and bone regeneration remain poorly understood. In the present study, we found that AS-IV treatment inhibited osteoclastogenesis, preserved preosteoclasts, and enhanced platelet-derived growth factor-BB (PDGF-BB)-induced angiogenesis. Additionally, AS-IV promoted cell viability, osteogenic differentiation, and angiogenic gene expression in bone marrow mesenchymal stem cells (BMSCs). The activation of AKT/GSK-3β/β-catenin signaling was found to contribute to the effects of AS-IV on osteoclastogenesis and osteogenesis. Furthermore, AS-IV accelerated bone regeneration during distraction osteogenesis (DO), as evidenced from the improved radiological and histological manifestations and biomechanical parameters, accompanied by enhanced angiogenesis within the distraction zone. In summary, AS-IV accelerates bone regeneration during DO, by enhancing osteogenesis and preosteoclast-induced angiogenesis simultaneously, partially through AKT/GSK-3β/β-catenin signaling. These findings reveal that AS-IV may serve as a potential bioactive molecule for promoting the coupling of osteogenesis and angiogenesis, and imply that AKT/GSK-3β/β-catenin signaling may be a promising therapeutic target for patients during DO treatment.
Keywords: angiogenesis; astragaloside IV; bone marrow mesenchymal stem cell; distraction osteogenesis; preosteoclast.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Enhanced Bone Regeneration by Schwann Cells through Coupling of Osteogenesis and Angiogenesis via β-catenin signaling in a Preclinical Model of Distraction Osteogenesis.Int J Med Sci. 2025 Jan 1;22(1):209-226. doi: 10.7150/ijms.100854. eCollection 2025. Int J Med Sci. 2025. PMID: 39744173 Free PMC article.
-
Accelerated Bone Regeneration by Adrenomedullin 2 Through Improving the Coupling of Osteogenesis and Angiogenesis via β-Catenin Signaling.Front Cell Dev Biol. 2021 Apr 14;9:649277. doi: 10.3389/fcell.2021.649277. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937244 Free PMC article.
-
Polydatin accelerates osteoporotic bone repair by inducing the osteogenesis-angiogenesis coupling of bone marrow mesenchymal stem cells via the PI3K/AKT/GSK-3β/β-catenin pathway.Int J Surg. 2025 Jan 1;111(1):411-425. doi: 10.1097/JS9.0000000000002075. Int J Surg. 2025. PMID: 39248296 Free PMC article.
-
Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects.Adv Pharmacol. 2020;87:89-112. doi: 10.1016/bs.apha.2019.08.002. Epub 2019 Dec 18. Adv Pharmacol. 2020. PMID: 32089240 Review.
-
Motivating role of type H vessels in bone regeneration.Cell Prolif. 2020 Sep;53(9):e12874. doi: 10.1111/cpr.12874. Epub 2020 Jul 19. Cell Prolif. 2020. PMID: 33448495 Free PMC article. Review.
Cited by
-
Smart osteoclasts targeted nanomedicine based on amorphous CaCO3 for effective osteoporosis reversal.J Nanobiotechnology. 2024 Apr 5;22(1):153. doi: 10.1186/s12951-024-02412-9. J Nanobiotechnology. 2024. PMID: 38580995 Free PMC article.
-
Advancements and challenges in pharmacokinetic and pharmacodynamic research on the traditional Chinese medicine saponins: a comprehensive review.Front Pharmacol. 2024 May 7;15:1393409. doi: 10.3389/fphar.2024.1393409. eCollection 2024. Front Pharmacol. 2024. PMID: 38774213 Free PMC article. Review.
-
A Novel Bioimplant Comprising Ad-BMP9-Transfected BMSCs and GelMA Microspheres Produced from Microfluidic Devices for Bone Tissue Engineering.J Tissue Eng Regen Med. 2023 Jun 19;2023:2981936. doi: 10.1155/2023/2981936. eCollection 2023. J Tissue Eng Regen Med. 2023. PMID: 40226408 Free PMC article.
-
Cyclic Distraction-Compression Dynamization Technique Enhances the Bone Formation During Distraction Osteogenesis.Front Bioeng Biotechnol. 2022 Jan 18;9:810723. doi: 10.3389/fbioe.2021.810723. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35118057 Free PMC article.
-
Immunomodulatory effects and mechanisms of distraction osteogenesis.Int J Oral Sci. 2022 Jan 24;14(1):4. doi: 10.1038/s41368-021-00156-y. Int J Oral Sci. 2022. PMID: 35067679 Free PMC article. Review.
References
-
- Mauffrey C, Barlow BT, Smith W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015;23:143–53. - PubMed
-
- Quinnan SM. Segmental Bone Loss Reconstruction Using Ring Fixation. J Orthop Trauma. 2017;31(Suppl 5):S42–s6. - PubMed
-
- Ilizarov GA. The principles of the Ilizarov method. Bull Hosp Jt Dis Orthop Inst. 1988;48:1–11. - PubMed
-
- Kong L-c, Li HA, Kang Q-l, Li G. An update to the advances in understanding distraction histogenesis: From biological mechanisms to novel clinical applications. Journal of Orthopaedic Translation. 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

