Transductive Transfer Learning for Domain Adaptation in Brain Magnetic Resonance Image Segmentation
- PMID: 33994917
- PMCID: PMC8116893
- DOI: 10.3389/fnins.2021.608808
Transductive Transfer Learning for Domain Adaptation in Brain Magnetic Resonance Image Segmentation
Abstract
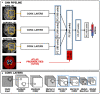
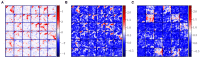
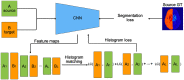
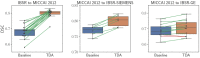
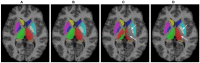
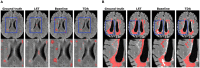
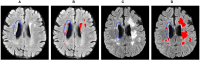
Segmentation of brain images from Magnetic Resonance Images (MRI) is an indispensable step in clinical practice. Morphological changes of sub-cortical brain structures and quantification of brain lesions are considered biomarkers of neurological and neurodegenerative disorders and used for diagnosis, treatment planning, and monitoring disease progression. In recent years, deep learning methods showed an outstanding performance in medical image segmentation. However, these methods suffer from generalisability problem due to inter-centre and inter-scanner variabilities of the MRI images. The main objective of the study is to develop an automated deep learning segmentation approach that is accurate and robust to the variabilities in scanner and acquisition protocols. In this paper, we propose a transductive transfer learning approach for domain adaptation to reduce the domain-shift effect in brain MRI segmentation. The transductive scenario assumes that there are sets of images from two different domains: (1) source-images with manually annotated labels; and (2) target-images without expert annotations. Then, the network is jointly optimised integrating both source and target images into the transductive training process to segment the regions of interest and to minimise the domain-shift effect. We proposed to use a histogram loss in the feature level to carry out the latter optimisation problem. In order to demonstrate the benefit of the proposed approach, the method has been tested in two different brain MRI image segmentation problems using multi-centre and multi-scanner databases for: (1) sub-cortical brain structure segmentation; and (2) white matter hyperintensities segmentation. The experiments showed that the segmentation performance of a pre-trained model could be significantly improved by up to 10%. For the first segmentation problem it was possible to achieve a maximum improvement from 0.680 to 0.799 in average Dice Similarity Coefficient (DSC) metric and for the second problem the average DSC improved from 0.504 to 0.602. Moreover, the improvements after domain adaptation were on par or showed better performance compared to the commonly used traditional unsupervised segmentation methods (FIRST and LST), also achieving faster execution time. Taking this into account, this work presents one more step toward the practical implementation of deep learning algorithms into the clinical routine.
Keywords: brain; deep learning; domain adaptation; magnetic resonance imaging; segmentation; sub-cortical structures; transductive learning; white matter hyperintensities.
Copyright © 2021 Kushibar, Salem, Valverde, Rovira, Salvi, Oliver and Lladó.
Conflict of interest statement
ÀR serves on scientific advisory boards for Novartis, Sanofi-Genzyme, Icometrix, SyntheticMR, and OLEA Medical, and has received speaker honoraria from Bayer, Sanofi-Genzyme, Bracco, Merck-Serono, Teva Pharmaceutical Industries Ltd, Novartis, Roche, and Biogen Idec. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
IAS-NET: Joint intraclassly adaptive GAN and segmentation network for unsupervised cross-domain in neonatal brain MRI segmentation.Med Phys. 2021 Nov;48(11):6962-6975. doi: 10.1002/mp.15212. Epub 2021 Sep 25. Med Phys. 2021. PMID: 34494276
-
Segmentation of white matter hyperintensities using convolutional neural networks with global spatial information in routine clinical brain MRI with none or mild vascular pathology.Comput Med Imaging Graph. 2018 Jun;66:28-43. doi: 10.1016/j.compmedimag.2018.02.002. Epub 2018 Feb 17. Comput Med Imaging Graph. 2018. PMID: 29523002
-
Two-stage adversarial learning based unsupervised domain adaptation for retinal OCT segmentation.Med Phys. 2024 Aug;51(8):5374-5385. doi: 10.1002/mp.17012. Epub 2024 Mar 1. Med Phys. 2024. PMID: 38426594
-
Quantifying deep grey matter atrophy using automated segmentation approaches: A systematic review of structural MRI studies.Neuroimage. 2019 Nov 1;201:116018. doi: 10.1016/j.neuroimage.2019.116018. Epub 2019 Jul 15. Neuroimage. 2019. PMID: 31319182
-
VoxResNet: Deep voxelwise residual networks for brain segmentation from 3D MR images.Neuroimage. 2018 Apr 15;170:446-455. doi: 10.1016/j.neuroimage.2017.04.041. Epub 2017 Apr 23. Neuroimage. 2018. PMID: 28445774 Review.
Cited by
-
Neuroimage analysis using artificial intelligence approaches: a systematic review.Med Biol Eng Comput. 2024 Sep;62(9):2599-2627. doi: 10.1007/s11517-024-03097-w. Epub 2024 Apr 26. Med Biol Eng Comput. 2024. PMID: 38664348
-
Exploring approaches to tackle cross-domain challenges in brain medical image segmentation: a systematic review.Front Neurosci. 2024 Jun 14;18:1401329. doi: 10.3389/fnins.2024.1401329. eCollection 2024. Front Neurosci. 2024. PMID: 38948927 Free PMC article.
-
Improving the Generalizability of Deep Learning for T2-Lesion Segmentation of Gliomas in the Post-Treatment Setting.Bioengineering (Basel). 2024 May 16;11(5):497. doi: 10.3390/bioengineering11050497. Bioengineering (Basel). 2024. PMID: 38790363 Free PMC article.
-
Survey of Transfer Learning Approaches in the Machine Learning of Digital Health Sensing Data.J Pers Med. 2023 Dec 12;13(12):1703. doi: 10.3390/jpm13121703. J Pers Med. 2023. PMID: 38138930 Free PMC article. Review.
References
-
- Bakas S., Reyes M., Jakab A., Bauer S., Rempfler M., Crimi A., et al. . (2018). Identifying the best machine learning algorithms for brain tumor segmentation, progression assessment, and overall survival prediction in the BRATS challenge. arXiv [Preprint]. arXiv:1811.02629.
-
- Campello M., Lekadir K. (2020). “Multi-centre multi-vendor & multi-disease cardiac image segmentation challenge (M&Ms),” in Medical Image Computing and Computer Assisted Intervention. (Lima: ).
LinkOut - more resources
Full Text Sources
Other Literature Sources

