TRPM4 Expression During Postnatal Developmental of Mouse CA1 Pyramidal Neurons
- PMID: 33994959
- PMCID: PMC8113704
- DOI: 10.3389/fnana.2021.643287
TRPM4 Expression During Postnatal Developmental of Mouse CA1 Pyramidal Neurons
Abstract
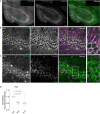
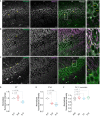
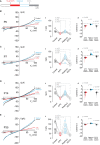
TRPM4 is a non-selective cation channel activated by intracellular calcium and permeable to monovalent cations. This channel participates in the control of neuronal firing, neuronal plasticity, and neuronal death. TRPM4 depolarizes dendritic spines and is critical for the induction of NMDA receptor-dependent long-term potentiation in CA1 pyramidal neurons. Despite its functional importance, no subcellular localization or expression during postnatal development has been described in this area. To examine the localization and expression of TRPM4, we performed duplex immunofluorescence and patch-clamp in brain slices at different postnatal ages in C57BL/6J mice. At P0 we found TRPM4 is expressed with a somatic pattern. At P7, P14, and P35, TRPM4 expression extended from the soma to the apical dendrites but was excluded from the axon initial segment. Patch-clamp recordings showed a TRPM4-like current active at the resting membrane potential from P0, which increased throughout the postnatal development. This current was dependent on intracellular Ca2+ (I CAN ) and sensitive to 9-phenanthrol (9-Ph). Inhibiting TRPM4 with 9-Ph hyperpolarized the membrane potential at P14 and P35, with no effect in earlier stages. Together, these results show that TRPM4 is expressed in CA1 pyramidal neurons in the soma and apical dendrites and associated with a TRPM4-like current, which depolarizes the neurons. The expression, localization, and function of TRPM4 throughout postnatal development in the CA1 hippocampal may underlie an important mechanism of control of membrane potential and action potential firing during critical periods of neuronal development, particularly during the establishment of circuits.
Keywords: CA1; ICAN; TRPM4; hippocampus; pyramidal neurons.
Copyright © 2021 Riquelme, Cerda and Leiva-Salcedo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Andersen P., Morris R., Amaral D., Bliss T., O’Keefe J. (2006). The Hippocampus Book. Illustrated Edition. Oxford: Oxford University Press.
-
- Bender R. A., Galindo R., Mameli M., Gonzalez-Vega R., Valenzuela C. F., Baram T. Z. (2005). Synchronized network activity in developing rat hippocampus involves regional hyperpolarization-activated cyclic nucleotide-gated (HCN) channel function. Eur. J. Neurosci. 22 2669–2674. 10.1111/j.1460-9568.2005.04407.x - DOI - PMC - PubMed
-
- Bovet-Carmona M., Krautwald K., Menigoz A., Vennekens R., Balschun D., Angenstein F. (2019). Low frequency pulse stimulation of Schaffer collaterals in Trpm4-/- knockout rats differently affects baseline BOLD signals in target regions of the right hippocampus but not BOLD responses at the site of stimulation. Neuroimage 188 347–356. 10.1016/j.neuroimage.2018.12.020 - DOI - PubMed
-
- Bovet-Carmona M., Menigoz A., Pinto S., Tambuyzer T., Krautwald K., Voets T., et al. (2018). Disentangling the role of TRPM4 in hippocampus-dependent plasticity and learning: an electrophysiological, behavioral and FMRI approach. Brain Struct. Funct. 223 3557–3576. 10.1007/s00429-018-1706-1 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

