Anacardium Occidentale L. Leaf Extracts Protect Against Glutamate/H2O2-Induced Oxidative Toxicity and Induce Neurite Outgrowth: The Involvement of SIRT1/Nrf2 Signaling Pathway and Teneurin 4 Transmembrane Protein
- PMID: 33995025
- PMCID: PMC8114061
- DOI: 10.3389/fphar.2021.627738
Anacardium Occidentale L. Leaf Extracts Protect Against Glutamate/H2O2-Induced Oxidative Toxicity and Induce Neurite Outgrowth: The Involvement of SIRT1/Nrf2 Signaling Pathway and Teneurin 4 Transmembrane Protein
Abstract
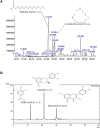
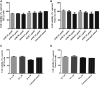
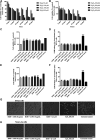
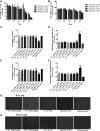
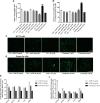
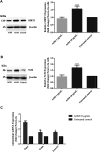
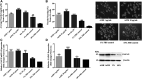
Neurodegenerative diseases are linked to neuronal cell death and neurite outgrowth impairment that are often caused by oxidative stress. Natural products, which have neuroprotective against oxidative stress and neurite outgrowth inducing activity, could be potential candidates for alternative treatment of neurodegenerative diseases. This study aims to investigate the neuroprotective effects and neuritogenesis properties of Anacardium occidentale leaf extracts in cultured neuronal (HT22 and Neuro-2a) cells. We found gallic acid, catechin and quercetin as the main compounds in A. occidentale extracts. The extracts have a protective effect against glutamate/H2O2-mediated oxidative stress-induced cell toxicity. The gene expression of cellular antioxidant enzymes (SODs, GPx and, GSTs) were up-regulated by this treatment. The treatment also triggered SIRT, Nrf2 proteins as well as the mRNA transcriptions of relevant anti-oxidation genes (NQO1, GCLM, and EAAT3). We demonstrated that the extracts promote antioxidant defense in neuronal cells via the SIRT1/Nrf2 signaling pathway. Moreover, the extracts increase neurite outgrowth and Ten-4 expression in Neuro-2a cells. However, the neuritogenesis properties did not occur, when Ten-4 expression was knocked down by corresponding siRNA. These results suggest that the leaf extracts have an interesting neuritogenesis and neuroprotective potential against glutamate/H2O2-mediated toxicity and could be a potential therapeutic candidate for neurodegenerative diseases.
Keywords: H2O2; Nrf2/SIRT1; anacardium occidentale; glutamate; neurite outgrowth; teneurin-4.
Copyright © 2021 Duangjan, Rangsinth, Zhang, Wink and Tencomnao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Neuroprotective Effects of Glochidion zeylanicum Leaf Extract against H2O2/Glutamate-Induced Toxicity in Cultured Neuronal Cells and Aβ-Induced Toxicity in Caenorhabditis elegans.Biology (Basel). 2021 Aug 19;10(8):800. doi: 10.3390/biology10080800. Biology (Basel). 2021. PMID: 34440032 Free PMC article.
-
Neuroprotective effects of oolong tea extracts against glutamate-induced toxicity in cultured neuronal cells and β-amyloid-induced toxicity in Caenorhabditis elegans.Food Funct. 2020 Sep 23;11(9):8179-8192. doi: 10.1039/d0fo01072c. Food Funct. 2020. PMID: 32966472
-
Acanthus ebracteatus leaf extract provides neuronal cell protection against oxidative stress injury induced by glutamate.BMC Complement Altern Med. 2018 Oct 16;18(1):278. doi: 10.1186/s12906-018-2340-4. BMC Complement Altern Med. 2018. PMID: 30326896 Free PMC article.
-
Leaf extract of Anacardium occidentale ameliorates biomarkers of neuroinflammation, memory loss, and neurobehavioral deficit in N(ω)-nitro-L-arginine methyl ester (L-NAME) treated rats.Biomarkers. 2023 May;28(3):263-272. doi: 10.1080/1354750X.2022.2164354. Epub 2023 Jan 25. Biomarkers. 2023. PMID: 36632742
-
Oolonghomobisflavans exert neuroprotective activities in cultured neuronal cells and anti-aging effects in Caenorhabditis elegans.Front Aging Neurosci. 2022 Sep 7;14:967316. doi: 10.3389/fnagi.2022.967316. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36158534 Free PMC article.
Cited by
-
Diet Supplementation with Prinsepiae Nux Extract in Broiler Chickens: Its Effect on Growth Performance and Expression of Antioxidant, Pro-Inflammatory, and Heat Shock Protein Genes.Animals (Basel). 2023 Dec 24;14(1):73. doi: 10.3390/ani14010073. Animals (Basel). 2023. PMID: 38200804 Free PMC article.
-
The Critical Role of Sirt1 in Subarachnoid Hemorrhages: Mechanism and Therapeutic Considerations.Brain Sci. 2023 Apr 18;13(4):674. doi: 10.3390/brainsci13040674. Brain Sci. 2023. PMID: 37190639 Free PMC article. Review.
-
Neuroprotective Effects against Glutamate-Induced HT-22 Hippocampal Cell Damage and Caenorhabditis elegans Lifespan/Healthspan Enhancing Activity of Auricularia polytricha Mushroom Extracts.Pharmaceuticals (Basel). 2021 Sep 29;14(10):1001. doi: 10.3390/ph14101001. Pharmaceuticals (Basel). 2021. PMID: 34681226 Free PMC article.
-
Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction.Plants (Basel). 2022 Dec 22;12(1):65. doi: 10.3390/plants12010065. Plants (Basel). 2022. PMID: 36616194 Free PMC article.
-
Neuroprotective Effects of Glochidion zeylanicum Leaf Extract against H2O2/Glutamate-Induced Toxicity in Cultured Neuronal Cells and Aβ-Induced Toxicity in Caenorhabditis elegans.Biology (Basel). 2021 Aug 19;10(8):800. doi: 10.3390/biology10080800. Biology (Basel). 2021. PMID: 34440032 Free PMC article.
References
-
- Bonaterra G. A., Schwendler A., Hüther J., Schwarzbach H., Schwarz A., Kolb C., et al. (2018). Neurotrophic, cytoprotective, and anti-inflammatory effects of St. John's wort extract on differentiated mouse hippocampal HT-22 neurons. Front. Pharmacol. 8, 955. 10.3389/fphar.2017.00955 - DOI - PMC - PubMed
-
- Chan G. K. L., Hu W. W. H., Zheng Z. X., Huang M., Lin Y. X. Y., Wang C. Y., et al. (2018). Quercetin potentiates the NGF-induced effects in cultured PC 12 cells: identification by HerboChips showing a binding with NGF. Evid. Based Complement. Alternat Med. 2018, 1502457. 10.1155/2018/1502457 - DOI - PMC - PubMed
-
- Duangjan C., Rangsinth P., Gu X., Zhang S., Wink M., Tencomnao T. (2019b). Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine 64, 153061. 10.1016/j.phymed.2019.153061 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

