Timing, Dosage, and Adherence of Antiretroviral Therapy and Risk of Osteoporosis in Patients With Human Immunodeficiency Virus Infection in Taiwan: A Nested Case-Control Study
- PMID: 33995032
- PMCID: PMC8121495
- DOI: 10.3389/fphar.2021.631480
Timing, Dosage, and Adherence of Antiretroviral Therapy and Risk of Osteoporosis in Patients With Human Immunodeficiency Virus Infection in Taiwan: A Nested Case-Control Study
Abstract
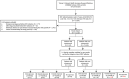
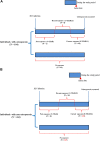
The progression of acquired immunodeficiency syndrome is delayed in patients with human immunodeficiency virus (HIV) infection receiving antiretroviral therapy (ART). However, long-term ART is associated with adverse effects. Osteoporosis is one of the adverse effects and is a multifactorial systemic skeletal disease associated with bone fragility and an increased risk of fracture. We performed a longitudinal, comprehensive, nested case-control study to explore the effect of ART on the risk of osteoporosis in 104 osteoporotic and 416 non-osteoporotic patients with HIV infection at their average age about 29 years old in Taiwan. Patients with history of ART, current exposure to ART, higher cumulative defined daily doses (DDDs), or higher ART adherence were at a higher risk of osteoporosis (p < 0.05). Patients receiving nucleoside/nucleotide reverse transcriptase inhibitor (NRTI)-containing regimen (zidovudine-lamivudine combination, lamivudine-abacavir combination, and abacavir alone) and protease inhibitor (PI)-containing regimen (lopinavir-ritonavir combination, ritonavir, and atazanavir) had a higher risk of osteoporosis (p < 0.05). Especially, patients receiving high doses of the PIs lopinavir-ritonavir combination had an increased risk of osteoporosis (p < 0.05). In conclusion, history of ART, current exposure to ART, higher cumulative DDDs, and higher ART adherence were associated with an increased risk of osteoporosis. Furthermore, NRTI- and PI-containing regimens and high doses of PIs lopinavir-ritonavir combination may be associated with an increased risk of osteoporosis in patients with HIV infection in Taiwan.
Keywords: HIV; adherence; antiretroviral therapy; dosage; osteoporosis; usage timing.
Copyright © 2021 Chiu, Liang, Li, Cheng, Chiou, Ho, Wu, Lin, Liao, Huang, Tsai and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Aberg J. A., Gallant J. E., Ghanem K. G., Emmanuel P., Zingman B. S., Horberg M. A., et al. (2014). Executive Summary: Primary Care Guidelines for the Management of Persons Infected With HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 58, 1–10. 10.1093/cid/cit757 - DOI - PubMed
-
- Ciccullo A., D'avino A., Lassandro A. P., Baldin G., Borghetti A., Dusina A., et al. (2018). Changes in Bone Mineral Density in HIV-Positive, Virologically Suppressed Patients Switching to Lamivudine/dolutegravir Dual Therapy: Preliminary Results from Clinical Practice. Infez Med. 26, 336–340. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

