SARS-CoV-2 Switches 'on' MAPK and NFκB Signaling via the Reduction of Nuclear DUSP1 and DUSP5 Expression
- PMID: 33995033
- PMCID: PMC8114414
- DOI: 10.3389/fphar.2021.631879
SARS-CoV-2 Switches 'on' MAPK and NFκB Signaling via the Reduction of Nuclear DUSP1 and DUSP5 Expression
Abstract
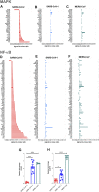
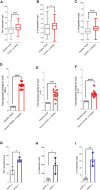
Mitogen-activated protein kinases (MAPK) and NF-kappaB (NF-κB) pathway regulate many cellular processes and are essential for immune cells function. Their activity is controlled by dual-specificity phosphatases (DUSPs). A comprehensive analysis of publicly available gene expression data sets of human airway epithelial cells (AECs) infected with SARS-CoV-2 identified DUSP1 and DUSP5 among the lowest induced transcripts within these pathways. These proteins are known to downregulate MAPK and NF-κB pathways; and their lower expression was associated with increased activity of MAPK and NF-κB signaling and enhanced expression of proinflammatory cytokines such as TNF-α. Infection with other coronaviruses did not have a similar effect on these genes. Interestingly, treatment with chloroquine and/or non-steroidal anti-inflammatory drugs counteracted the SARS-CoV-2 induced reduction of DUSP1 and DUSP5 genes expression. Therapeutically, impeding this evasion mechanism of SARS-CoV-2 may help control the exaggerated activation of these immune regulatory pathways during a COVID-19 infection.
Keywords: COVID-19; DUSP1; DUSP5; MAPK; NFkB; SARS-CoV-2; chroloquine.
Copyright © 2021 Goel, Saheb Sharif-Askari, Saheb Sharif Askari, Madkhana, Alwaa, Mahboub, Zakeri, Ratemi, Hamoudi, Hamid and Halwani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

